More than a thousand different versions of the periodic table have been created since Dmitri Mendeleev drew the first 150 years ago – but why is one version more familiar to us than practically every other? Robert P Crease finds out
Early in 1869 the Russian chemical physicist Dmitri Mendeleev was growing increasingly frustrated while preparing the second volume of his textbook Principles of Chemistry. Though his first volume had been 600 pages long, Mendeleev had managed to cover barely eight of the 60 or so known elements in it. Facing a publisher’s deadline for the sequel before a looming trip to Europe, he decided to sketch out a table, putting the elements in columns and rows, ordering them by atomic weight in a way that showed their chemical similarities. He dated the table 17 February in the Julian calendar then used in Russia (corresponding to 1 March in the Gregorian calendar used throughout Europe).
Mendeleev was not completely satisfied with this table, and would go on to create about 60 more versions of the scheme. But his 17 February table became the basis for the one that now appears in the pages of every chemistry textbook and hangs in nearly every chemistry classroom around the world. But why did this table rise to fame, rather than the thousand or so other versions that one can find in the Internet Database of Periodic Tables? It includes many that look similar but also spiral, helical, circular and even 3D tables. The standard, textbook answer is that Mendeleev’s was the first table that graphically tried to display the chemical relations between all known elements ordered by atomic weight. But the real reason is more complicated.
One person with the answer is Ann Robinson, a science historian who works in the Widener Library at Harvard University in the US. Robinson is far from the first person to study the history of the table, but she is one of the most recent, having received her PhD on the subject last year from the University of Massachusetts in Amherst. Her thesis is timely: this month is the 150th anniversary of Mendeleev’s 17 February table, and July sees the centenary of the International Union of Pure and Applied Chemistry (IUPAC), which oversees global nomenclature and standards in chemistry.
Weighty matters
I recently visited Robinson at the Widener, which is the geographical and intellectual heart of the Harvard campus. Meeting me in the lobby of this imposing, colonnaded building, Robinson showed me one of the library’s treasures – a Gutenberg Bible, prominently displayed in an ornate chamber and one of just 49 surviving copies of this historically significant book.
By the 1860s, Robinson explained, chemists had settled on atomic weights as the most important characteristic for ordering the elements. Some chemists had even noticed that, when sequenced that way, groups of elements had similar properties. A few tried to express this insight systematically, including the British scientist John Newlands. Writing in a series of short articles published in Chemical News in the early 1860s, he proposed – and graphically illustrated – a “law of octaves”, whose principle was that every eighth element in his list exhibited similar chemical behaviour. But others ridiculed Newlands’ law, with many chemists thinking that he had pulled it out of thin air. It didn’t help either that Newlands had compared the relationships of elements to those of notes in a musical octave, a link that seemed whimsical to everyone but Newlands himself.
Over in Russia, meanwhile, and initially ignorant of Newlands’ work, Mendeleev was working at St Petersburg University, where he had been appointed a professor in 1864. He had become dissatisfied with existing chemistry textbooks, which divided elements into either metals or non-metals and classified them by their “valency”, or ability to combine with other elements. As the Princeton University science historian Michael Gordin recounts in his recently revised book A Well-Ordered Thing: Dmitri Mendeleev and the Shadow of the Periodic Table, Mendeleev set out to write his own textbook, sending Volume 1 to the publisher in January 1869.

But having covered only eight of the 63 known elements, Mendeleev began to condense his presentation in Volume II in the face of a contract deadline and page limitations, seeking to clarify the remaining elements in a way that would draw out their relationships. And so it was that on 17 February 1869 Mendeleev sent the Russian Chemical Society a single-page diagram, entitled “An attempt at a system of elements, based on their atomic weight and chemical affinity”. The scheme, Gordin notes, “arose out of the need for a pedagogical ‘classification’ [for] presenting material to beginning chemistry students”. Mendeleev then submitted a paper to the society and in 1869 published an abstract of it along with a version of the table in Zeitschrift für Chemie (12 405).
Robinson pulled out a copy of Mendeleev’s original diagram. Consisting of six columns of anywhere from two to 19 elements, it looked nothing like the modern periodic table we know and love. “You have to rotate it 90° clockwise, and imagine the elements flipped left to right, and the entire table unsquished,” she explained. When I did, it indeed looked roughly similar to our familiar table.
Fill the blanks
But Mendeleev was still dissatisfied with his new scheme. One troublesome feature, Robinson explained, was that in several instances, placing elements by their chemical behaviour collided with their placement by atomic weight. Tellurium (Te), for example, to which Mendeleev hesitantly assigned an atomic weight of “128?”, had to be placed before iodine (I), which had an atomic weight of 127. (We now know that the atomic weight is 127.6 for Te and 126.9 for I.)

Another puzzling feature, Robinson said, was that the table literally had holes into which Mendeleev had inserted question marks. In the table published with the abstract, these locations are for elements beneath boron (B), aluminium (Al), manganese (Mn) and silicon (Si) – the so-called rare earths, with approximate atomic weights of 45, 68, 70 and 180. Mendeleev decided that these holes weren’t the result of inadequacies in his classification scheme, but would be filled by hitherto undiscovered elements. Indeed, Mendeleev was so confident the new elements existed that he described their chemical behaviour and even gave them names by attaching the prefix “eka” (from the Sanskrit for “proto”) to those they most closely resembled. The element below aluminium, for example, became eka-aluminium.
A further curiosity of Mendeleev’s early tables, to us, is that one slot was occupied by an element labelled “Di” with an atomic weight of 95. As Robinson explained to me, that referred to didymium, which later turned out not to be an element but a combination of praseodymium (Pr) and neodymium (Nd). It’s a real substance, though, used in safety glasses to block out yellow light.
The first support for Mendeleev’s vision came in 1875 when the French chemist Paul Émile Lecoq de Boisbuadran discovered eka-aluminium, which he renamed gallia (in honour of the Latin name for the region of Gaul) – gallium (Ga) in modern English. The finding attracted attention to Mendeleev’s scheme, although most chemists were still unconvinced. As Robinson put it, the prediction was seen as “a lucky guess, a fluke”.
Then, four years later, the Swedish chemist Lars Fredrik Nilson discovered eka-boron – what we now call scandium (Sc). “Could he be lucky twice?” Robinson pointed out. “People thought, ‘maybe there’s something to this after all!’” More convincing was that Mendeleev had not simply predicted these elements, but also accurately forecast their properties and atomic weights. Further evidence in support of his table came in 1886 when eka-silicon (now germanium, Ge) was discovered, although the world had to wait until 1937 for the discovery of eka-manganese, which is now known as technetium (Tc) and one of whose isotopes is vital in medical physics.
Challenges ahead
Mendeleev’s table was thus the first time that a chemical classification scheme had been used to predict yet-unknown elements, and the Russian’s confidence in his basic scheme grew. Robinson pulled out a copy of the first English translation of his Principles of Chemistry, which was published in 1891 and based on the book’s fifth Russian edition, revised by Mendeleev. The table in that edition had lots of other un-named blank spaces, including the place where radium (Ra) would go 20 years later. “The chief theme of this work,” Mendeleev wrote in the preface, is the “philosophical principles” of chemistry. In it, he sought to “cast aside classical illusions”, and present the material “which not only gives mental satisfaction but is also practically useful”. He said his presentation was based on “the law of periodicity” of chemical elements and declared that his table placed them “in series, groups, and periods”. This version of the table resembled the one Robinson had shown me earlier, but with the elements flipped.
Mendeleev’s system was not entirely without problems, however. In 1870 he’d already had to gamble by doubling the atomic weight of uranium (U) from about 120 to 240 to make it fit (it’s actually about 238). Mendeleev also had trouble figuring out what to do with the lanthanides – a band of 15 elements including lanthanum (La), cerium (Ce) and neodymium (Nd) – and stuck them below the main part of the table. They were chemically similar to each other, but didn’t fit with the rest of the table. “They still don’t, and are still down there at the bottom,” Robinson wryly noted.
One troublesome feature for Mendeleev was that in several instances, placing elements by their chemical behaviour collided with their placement by atomic weight
Soon there were more disturbing events. One problem was J J Thomson’s discovery of the electron, the first subatomic particle, in 1897. Mendeleev did not think it existed. But some scientists tried (unsuccessfully) to incorporate it into the periodic table, giving it an atomic weight of zero. Another near-simultaneous event was Henri Becquerel’s discovery of radioactivity, which led to the conception of radioelements – radioactive substances that were thought to be elements. Chemists now had to postulate the existence of elements whose properties could not yet be determined, and possibly never could be. Mendeleev was also unconvinced by the idea of radioactivity, and in 1902 wrote an article proposing a “chemical conception” of the ether that would account for it. Even Marie Curie thought radioactive substances were impossible to characterize as chemical elements on account of “their destruction being too rapid”, and proposed the development of a “chemistry of the invisible”. Some periodic tables of the early 20th century did not include radioactive substances at all.
Radio-elements were puzzling for other reasons as well. They seemed to indicate the existence of substances with different chemical properties that looked like they occupied the same place on the periodic table, and substances with the same chemical behaviour that looked like they occupied two different places. “Elements aren’t supposed to do that,” Robinson said. “It was supposed to be one element per square!” But if radioactive elements challenged early visions of the periodic table, it also facilitated the discovery of new elements of this kind.
By 1914 things had pretty much been cleared up. Ernest Rutherford had discovered the atomic nucleus three years before, and – thanks to the notion of quantum physics – Niels Bohr had made Rutherford’s scheme workable. Frederick Soddy then demonstrated the existence of isotopes: atoms of one particular element with the same number of protons but different numbers of neutrons. The notion solved the placement problem, because all isotopes of an element can be put in the same square of the periodic table, with the atomic weight of an element – what’s now also called the relative atomic mass – being the average mass of all atoms of the element, taking into account their different abundances. Henry Moseley’s X-ray studies showed that the “atomic number” of an element is not an arbitrary number but associated with a specific measurable property of the atomic nucleus. It is the number of protons in an atom and thus its numerical place on the periodic table when elements are arranged by atomic weights
Moseley’s work cemented the justification for Mendeleev’s scheme, and among other things definitively established the legitimacy of placing tellurium (52 protons) before iodine (53 protons) on the periodic table. Yet the increasing primacy of atomic number over atomic weight, Robinson said, was accompanied by a fear among chemists that physics was encroaching on their role in characterizing elements.
The road to fame
In her PhD dissertation, Robinson traces what followed. After 1869 many chemists produced versions of Mendeleev’s table, and teachers found such tables useful. The first textbooks to include a periodic table were published in the 1870s, but the first version of the table that we are most familiar with did not appear until 1923 when the US chemist Horace G Deming published a textbook called General Chemistry: an Elementary Survey Emphasizing Industrial Applications of Fundamental Principles.
Robinson showed me a second edition of Deming’s book, which wasn’t aimed at academic researchers. “It was geared towards students who wouldn’t necessarily go much further in chemistry, or who would go into industry,” she explained. The textbook proved a huge hit and went through six editions – the last in 1952 – popularizing Deming’s revised version of Mendeleev’s table. Chemical companies also began using it in their material and, by 1928 the American pharmaceutical giant Merck had shrunk the table and distributed it in letter size and index-card size. In 1934 it was the first periodic table to be incorporated into the CRC Handbook of Chemistry and Physics – a standard reference manual. “Those events made it the table,” Robinson said. “It was a pragmatic system that was extremely useful in chemical education.” The key point of her dissertation, in fact, is that “pedagogy played a more important role than research did in the development of the form of the periodic table”.
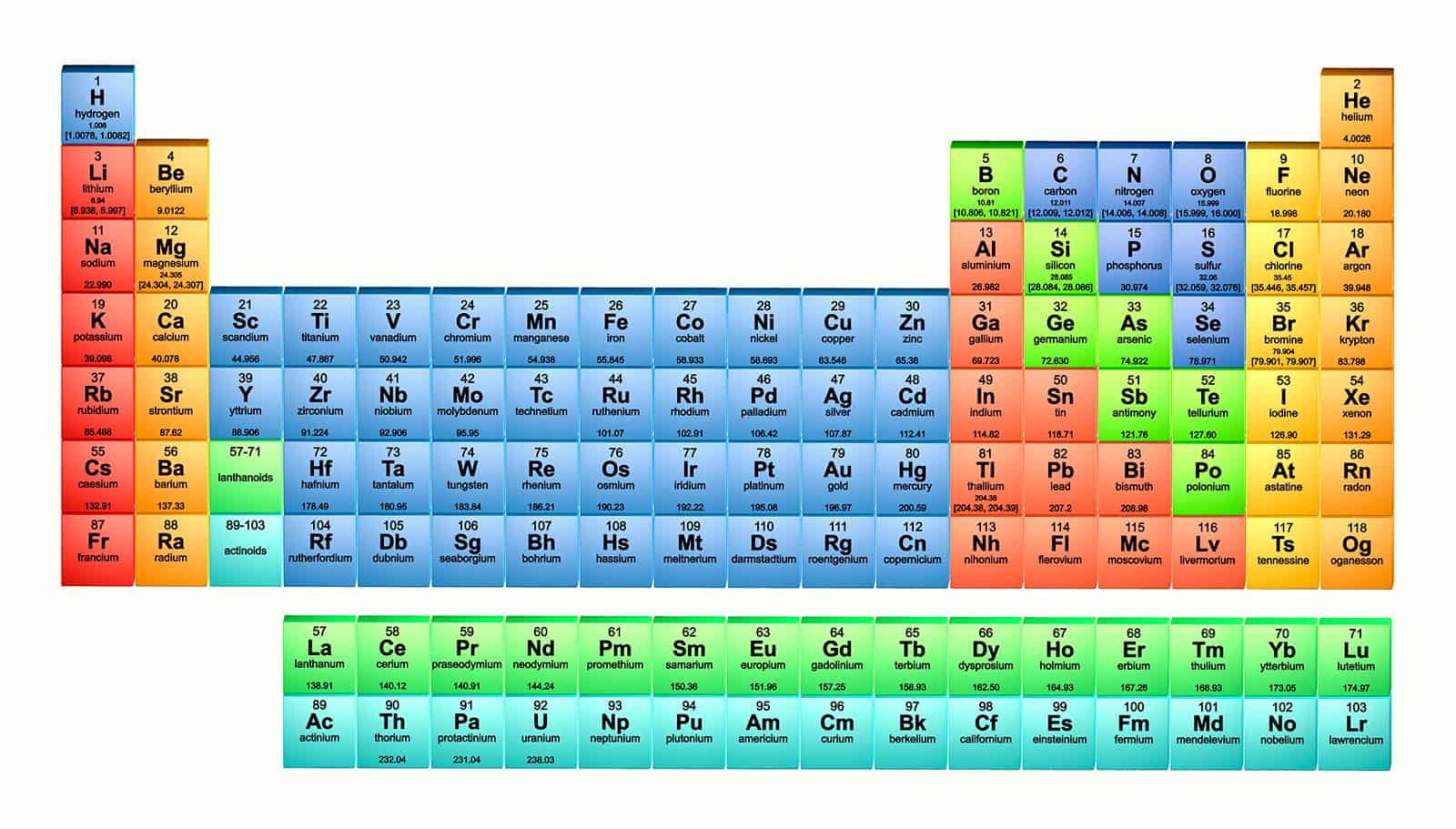
The table has even proven resilient enough to survive the discovery, over the last 25 years, of elements whose atomic number is over 110, filling out the remaining space in the table’s seventh row. “One of the great features of the periodic table,” Robinson reminded me, “is its elasticity, its ability to accommodate elements that Mendeleev would never have expected, such as synthetic elements.”
But the periodic table, whether Mendeleev’s or the others, did not solve one problem. What, finally, is an element? It is one example of a scientific issue that cannot be resolved by more research, but requires reflection on basic principles that guide theory and experiment. Answering the question falls within the bounds of philosophy of science. Indeed, some philosophers have wondered whether the “superheavy” elements at the end of the periodic table (those with atomic weights of 104 and over) can really be called elements. Given that they are not found in nature, can only be made in labs and disintegrate almost immediately, they raise serious questions about whether they even “exist”.

Scientists celebrate 150 years of the periodic table at UNESCO headquarters in Paris
Another philosophical issue that the periodic table raises is the boundary between physics and chemistry. Is an element a species of something whose fundamental bits share the same structure, which is how physicists think of them? Or is an element something with properties like colour and smell that can be experimented on – the chemists’ view? Physicists generally picture elements as invisible things that can’t be seen and remain the same when combined, while chemists think about their elements as real substances you can hold in your hand, like a lump of tungsten. Both the physicists’ and the chemists’ views seem integral to the idea of an element, though they involve different pictures. Mendeleev had already encountered this problem, and those who followed often tried to solve it with such distinctions as pure and simple substances, or chemical element and elementary substance. Such distinctions represent the practical divide between chemistry and physics.
The issue persists today, with even IUPAC refusing to take a stand on the definitive periodic table. Its Compendium of Chemical Technology – colloquially called the Gold Book because that’s the cover’s colour – gives not one definition of an element but two. The first defines an element as “all atoms with the same number of protons in the atomic nucleus”, while the second defines it as “a pure chemical substance composed of atoms with the same number of protons in the atomic nucleus”. But 150 years after Mendeleev’s first periodic table, the fact that this distinction still persists is, for Robinson, a reaction to the intrusion of physics into chemistry. “You physicists can play with our elements all you want,” she warned. “But there’s still a chemical element in there that you cannot take away!”