Researchers at ETH Zurich in Switzerland have tracked the ultrafast movements of electrons in liquid water for the first time – an important step towards understanding the fine details of how chemical and biochemical reactions begin in liquid environments.
According to the classical Bohr model of the atom, an electron takes roughly 150 attoseconds (10-18s) to orbit the proton in a hydrogen atom. Measurements on a timescale of a few dozen attoseconds have been possible for several years now, notes team leader Hans Jakob Wörner of ETH’s Laboratory of Physical Chemistry, and even at lower resolutions of 10 femtoseconds (10-15 s) it is already possible to observe certain subatomic-scale processes – up to and including the breaking of chemical bonds. “Electron movements are the key events in chemical reactions,” he says. “That’s why it’s so important to measure them on a high-resolution time scale.”
Wörner’s team were among the first to detect electron movements at the attosecond scale. However, because the wavelength of their attosecond pulses is in the extreme ultraviolet (XUV) range, which is easily absorbed by air, they had to carry out their measurements in a vacuum chamber. This restriction meant that they were only able to perform attosecond measurements on molecules in the gaseous state.
Novel measuring scheme
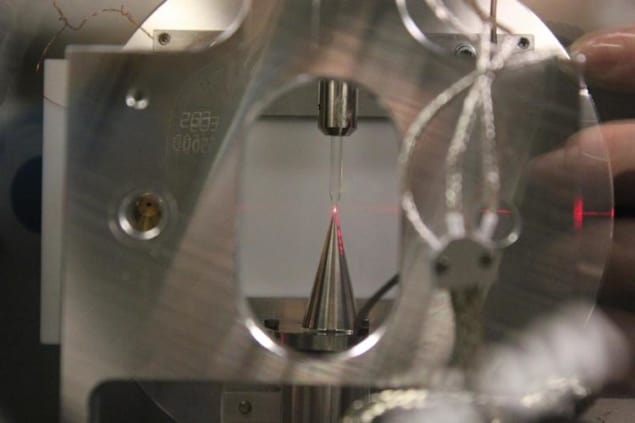
The researchers have now developed a novel measuring scheme that relies on comparing photoemissions (that is, emissions of electrons prompted by light striking a material) from liquid water with those of water vapour. In their experiment, they inject a microscopic jet of liquid water into their vacuum chamber through a quartz nozzle with an inner diameter of roughly 25 mm. This jet flows smoothly (laminar flow) for a few millimetres before breaking up into droplets, which then freeze upon contact with a liquid-nitrogen cold trap. “The laminar-flow region of the microjet allows us to study liquid water in a quasi-equilibrium state, very close to room temperature,” Wörner explains.
To make their attosecond measurements, the team focus an XUV train of attosecond pulses (obtained from a 30-femtosecond near-IR laser pulse in an argon gas cell) onto the evaporating water. In addition to this XUV pulse train, they also focus a strongly attenuated replica of the IR pulse onto the liquid microjet. This set-up allows them to detect electrons photoemitted from the liquid and gas phases at the same time.

Industrial lasers generate attosecond light pulses
The ETH Zurich researchers observed that photoelectrons from liquid water molecules are emitted 50-70 attoseconds after those emitted from gaseous water molecules. This time difference stems from the fact that the molecules in liquid form are surrounded by other water molecules, Wörner explains. This affects the molecules’ electronic structure, producing a measurable time delay.
An important step forward
Going from measurements in gases to measurements in liquids is an important step forward, since most chemical reactions – especially those that are biochemically interesting – take place in liquids, Wörner notes. Reactions such as photosynthesis in plants and the biochemical processes on our retina that allow us to see are also triggered by light, just like photoemission in water – which is also the dominant source of DNA damage due to X-rays or other ionizing radiation. “With the help of attosecond measurements, scientists should gain new insights into the most elementary steps of these processes in the coming years,” he says.
The research is detailed in Science.