Scientists are seeing the human body in a new light, thanks to a unique synchrotron-imaging technique, as Jon Cartwright discovers
In this age of information, we expect to have knowledge at our fingertips. If we’re looking to obtain a first impression of someone, many of us head straight to their social-media pages. If we want to understand a new topic, we don’t buy a textbook – most of the basics are waiting for us on Wikipedia. And if we want to explore a new city, we can do much of it by moving around in Google Earth. Information that was once costly or exclusive is now free to all.
But what about medical images? Suppose you want to explore what a real human heart looks like, from the entire organ down to the smallest blood vessels. Currently, for most of us, that’s impossible. True, a heart surgeon could obtain radiological images of a patient’s heart, and order biopsies of specific volumes. But even then, the doctor will be easily frustrated by the limitations of individual imaging methods.
Clinical computed tomography (CT), which uses X-rays to build up 3D images slice by slice, is restricted to millimetre resolution. So too is magnetic resonance imaging (MRI), which peers inside the body using magnetic fields and radio waves. Microscopy of biopsies, meanwhile, is usually limited to millimetre-sized volumes. The dream of seeing an organ – or the entire human body – with micron or near-micron resolution has simply been out of the question, whether you are a specialist or not.
Not any more. For the last two years, dozens of scientists in Europe have been busy compiling the most detailed 3D views of real organs ever seen. Like a Google Earth of the human body, the Human Organ Atlas, as the team’s project is known, is both simple and astonishing. Its goal is to create a freely accessible, online image bank of highly “zoomable” human organs, revealing everything from their biggest features (on the scale of centimetres and metres) all the way down to micro-scale structures.
The project has already led to the creation of 3D images of lungs, a brain, a heart, a kidney, a spleen and a liver. By 2025 the Human Organ Atlas team wants to have imaged an entire human torso and, not too far beyond that, an entire human body. The work is impressive for scientists and non-scientists alike – so much so that the project is being bankrolled by some high-profile funding agencies in the UK, EU and US. Even Google has taken an interest.
One scientist who has been collaborating on the project is Danny Jonigk, a lung pathologist at Hannover Medical School in Germany. He feels as if he has spent his entire career doing research under candlelight, only for someone “to suddenly switch the lights on”. Then there’s Daniyal Jafree, a medical student at University College London (UCL) in the UK, who’s doing a PhD in kidney imaging. When he heard what was being developed elsewhere at UCL, Jafree couldn’t quite believe it. “I thought that sounds ambitious,” he says. “Then I saw the images.”
X-rays at your service
The Human Organ Atlas project wouldn’t be possible without physics. It began at the European Synchrotron Radiation Facility (ESRF) in Grenoble, France, which has been one of the world’s foremost X-ray light sources since it opened more than 30 years ago. Unlike the X-rays delivered by a clinical CT scanner, synchrotron X-rays have high energy and a high spatial coherence. That means their waveforms remain very much in phase with one another as they propagate, allowing researchers to exploit minute changes in X-ray phase to produce tomographic (section-by-section) 3D images of very high detail and contrast (see box below).
For many years, this phase-contrast X-ray technique has delivered incredible reconstructions of biological specimens. In 2011, for example, ESRF beamline scientist Paul Tafforeau helped produce what is still the most detailed scan ever of the inside of a skull of an early human ancestor, Australopithecus sediba. More recently, he has produced scans of small dinosaur fossils, ancient human teeth and even mummified crocodiles.

Then, in 2020, two things happened. The first was that the ESRF finished commissioning a new, “fourth-generation” source, making it the world’s brightest synchrotron lab. More than a decade in planning and construction, the Extremely Brilliant Source (EBS) delivers X-rays that are 100 times brighter than before, and 100 times more coherent in the transverse (horizontal) plane, making them almost laser-like at low energies. The EBS has done wonders for tomographic imaging, enabling users to scan bigger objects, in more detail and at a greater range of scales.
The second big event of 2020 was, of course, the COVID-19 pandemic. For many scientists, the pandemic brought research to a full stop. Not for Tafforeau. Unexpectedly, he received a call from Peter Lee, a regular ESRF tomography user at UCL, who in turn had been approached by Jonigk. Could the ESRF be of help, Lee wondered, in reconstructing lung tissue samples from people who had died after catching COVID-19? It was a great question and almost overnight Tafforeau switched from study-ing ancient fossils to human organs.
“The COVID-19 pandemic changed a lot of things for many people,” Tafforeau recalls. “I realized that several imaging techniques that we originally developed for palaeontology could open access to a new level of imaging precision on complete human organs. Then, while developing the techniques further, we realized that it may be a game-changer for biological imaging in general.”
Swiftly, Lee composed an international, multidisciplinary team to see what could be done: synchrotron imaging scientists at UCL and the ESRF; mathematicians and computer scientists at UCL; medical scientists at Hannover Biobank, as well as the universities of Mainz and Heidelberg in Germany. As the apparent potential of the new tomographic imaging grew, so did the breadth of the collaboration: it now includes more than 50 people.
The scientists called the technique hierarchical phase-contrast tomography (HiP-CT), thanks to its ability to provide 3D reconstructions of entire intact organs that can then be explored anywhere down to the cellular level. As a result, the technique bridges the gap in scales between clinical CT and MRI, and the microscopy of biopsies. In November 2021 the project was formalized as the Human Organ Atlas, with a goal to provide a reference database of organ imagery that is accessible to all.
Hierarchical Phase-Contrast Tomography (HiP-CT) in a nutshell
Most simple imaging methods – including conventional computed tomography (CT) – involve measuring the loss of intensity (the attenuation) of an electromagnetic wave as it passes through a sample. In 1953, however, the Dutch physicist Frits Zernike won the Nobel Prize for Physics for developing an alternative – and potentially more illuminating – imaging method that involves measuring shifts in the phase of electromagnetic rays.
Zernike’s “phase-contrast” microscopy was initially fit only for visible light. But in 1965 it started being extended to X-rays too thanks to the work of Ulrich Bonse and Michael Hart – two physicists at Cornell University in the US – who used a crystal interferometer to convert phase changes into interference patterns.
Limitations with interferometers meant that phase-contrast X-ray imaging of biological samples had to wait until the 1990s through the efforts of Atsushi Momose at Hitachi and Tohoru Takeda at the University of Tsukuba, Japan, and others. At roughly the same time, Anatoliy Snigirev and others at the European Synchrotron Radiation Facility in Grenoble, France, realized they could deduce phase changes without an interferometer, simply from the interference of highly coherent synchrotron X-rays in free space. By combining many propagation phase-contrast 2D images in CT mode, they were able to produce 3D reconstructions of small biological samples with far more detail than that available from clinical CT scanners.
With the upgrade of the ESRF to a “fourth-generation” X-ray source in 2020, “hierarchical” phase-contrast CT (HiP-CT) became possible. The lab’s ultra-coherent X-rays provide information on phase changes over very long propagation distances up to 40 m, allowing samples of up to 2.5 m × 1.5 m in size – including human organs, torsos, even entire bodies – to be imaged in 3D at micron resolution.
The atlas in action
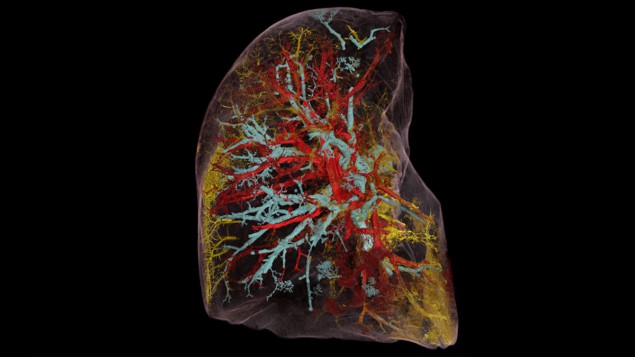
A video of a human brain, as imaged by HiP-CT, gives an impression of the technique’s capabilities (figure 1). It starts off conventionally enough, moving through cross sections of the entire organ. Here the brain looks like it does with a clinical CT scan, albeit at some 50 times the resolution. The various lobes are clearly visible, as are some of the external blood vessels. Then the “camera” zooms in to the back of the brain, the cerebellum, perfectly transitioning from big to small.
At 5 μm resolution, the smallest features of white and grey matter come into view; at 2.5 μm resolution, the tiniest blood vessels can be discerned. Even pyramid-shaped cells can be seen, known as Purkinje neurons, which are largely responsible for human motor function. Finally, the view retreats and the reconstruction morphs to depict blood vessels only. Now the incredible density and complexity of the brain’s “vasculature” become apparent. As the system that delivers and receives the oxygen, glucose and metabolic waste, it keeps every one of us alive and thinking.
The HiP-CT video looks like the cutting-edge CGI you see in sci-fi blockbusters – yet it is perfectly real. What’s more, as all the raw data have been collected and post-processed, it’s possible for scientists to explore different parts of the brain at will. In fact, the sheer wealth of information in the imagery is so great that interpreting it is a major problem in itself. The team divides the work, with Tafforeau in charge of reconstructing the images and the UCL team trying to make sense of them.

“It’s a little bit overwhelming, like being a kid in a sweetie shop,” admits Claire Walsh, a biophysicist in UCL’s computational analysis team. “Medics and histologists can tell us when something looks weird, but we have to quantify that: exactly how weird?” One example is the size of alveoli, which has in the past been used to indicate the seriousness of lung disease. Previously, says Walsh, the alveoli were assumed to be roughly spherical, like grapes on a vine. But the new technique reveals them to be more irregular.
As a result, the researchers have had to define new parameters, with input from their medical collaborators, to capture the potential of the new information. Joseph Jacob, a chest radiologist who joined the UCL team early in the pandemic, stresses the scale of the interpretation challenge. “When I first saw the images, I felt amazement and apprehension – probably apprehension more than amazement,” he says. “It was definitely what I wanted to work on, but the complexity of labelling it – obviously it would only be possible with computer science.”
Fortunately, Jacob knows how vital the image-processing of X-ray data is, having developed algorithms to stitch together hundreds of CT images to see the lung in detail. He believes the reward now is well worth the labour. “[This new technique] is going to show us things we never knew existed,” he says, which could be vital given how medicine is a very “organ-centric” discipline. “As a chest specialist, I just look at the lungs – I don’t look at the heart, for instance. But disease doesn’t necessarily work that way. If you could image a whole torso, you could understand how disease is affecting other organs; it would be a much more rounded approach.”
The way ahead
As things stand, almost all the organs in the atlas have been imaged at the ESRF’s long-serving BM05 beamline. In December 2021, however, the team acquired its first HiP-CT images at BM18 – a new ESRF beamline that has been designed to maximize the benefits of the EBS for microtomographic images of large objects. Although the beamline won’t be fully operational until the end of 2022, it will eventually be able to image a torso – and even an entire human body.
Imagine one day being able to explore, in virtual reality, human bodies of all ages, backgrounds, states of health and disease. As Lee points out, the damage wrought by new diseases could then be easily compared with that of existing conditions, to indicate possible known methods of treatment. People could see what sort of processes might be going on inside themselves. Medics could entertain pure curiosity, without having to resort to the knife.

From whole-organ to cellular resolution: synchrotron X-ray images reveal COVID-19 lung damage
We are not there yet, but preliminary images have already given some indication of the benefits of the large-scale, detailed view of HiP-CT. Reconstructions of several lungs from COVID-19 victims have revealed heterogeneous damage that appeared on previous clinical CT scans merely as a fuzzy, ground-glass texture (Nature Methods 18 1532). The result is helping to determine whether it is the connectedness of lung damage, or the sheer amount of it, that is the cause of death by the virus.
Meanwhile, Jafree is keen to find out if HiP-CT can help us to give us a better understanding of the kidneys, the organs he specializes in. We know that the number of blood-vessel networks, or glomeruli, is a proxy for general kidney function. But no-one knows how losing some of these networks affects those that remain, or whether their volume or shape affects kidney health. “HiP-CT allows us to look at things in a different way,” says Jafree. “It also encourages [students] like me to learn some of the image-analysis techniques. We need that expertise to generate something meaningful for biology and medicine – and we have an incentive now.”

Sarah Teichmann, a cellular geneticist at the Wellcome Sanger Institute in Hinxton, UK, says she was “blown away” by the first HiP-CT images she saw, letting her view the cellular structures inside organs in exquisite detail, before zooming out to see the whole tissue. “Not only do these images and videos give a new appreciation of the beautiful complexity of the human body,” she says, “they are also stocked full of information about how our bodies work.”
Teichmann believes that the whole-organ or whole-body approach could benefit our understanding of diseases such as cancer. She also reckons that the Human Organ Atlas project ties in well with the Human Cell Atlas – an international consortium that she co-founded to create a comprehensive reference map of all human cells. “[It] could help us to see where these cell types – which we characterize at the molecular level – fit into the bigger picture of the organ. This will help to bridge the gap between cells and systems, painting a more holistic picture of the human body.”
Beautiful times
Alongside the huge scientific impact of this imaging technique, there is also an inherent beauty to the images taken by the Human Organ Atlas. In December 2021 National Geographic magazine picked a HiP-CT image of a lung as one of its favourite science images of the year. Francesco Sette, the physicist who has been director-general of the ESRF since 2009, has even compared the advancement of the technique with Leonardo da Vinci’s anatomical drawings of the early 16th century.
Those drawings gave unprecedented insights into the workings of the human body, especially its biomechanics. It is not yet clear what the ramifications of the Human Organ Atlas will be, although the concept is proving popular. The collaboration’s Nature Methods paper has been downloaded more than 50,000 times, and is in the top 1% of Nature articles in terms of its Altmetric score, or reach.
The project is also gaining some serious backers, not least a $2.75m donation from the Chan-Zuckerberg Initiative (CZI), which was set up by Facebook founder Mark Zuckerberg and his wife Priscilla Chan. The CZI is independent of Facebook – which may be a good thing, as the Atlas team is just beginning a collaboration with Google to make its database available to the public. According to Lee, the plan is to create something like an anatomical version of Google Earth, with 3D “satellite” resolution of 40 μm resolution for a whole organ, and a 3D “street view” resolution down to 1 μm to expose individual cells.
After Google Earth, Google Maps and Google Sky, perhaps it is fitting that one day we will have a Google Body search tool too.



