
Glioblastoma is the most common and aggressive malignant brain tumour in adults. Treatment relies mainly on surgical resection, but complete resection is difficult and improved methods for detecting regions of brain tumour are key to a positive outcome. Researchers in Italy have performed a proof-of-concept study showing that a combination of three different optical spectroscopic techniques has potential to improve the in vivo detection and delineation of malignant brain tumours and to guide their surgical removal.
The team used a multimodal optical fibre probe combining fluorescence, Raman and reflectance spectroscopy for in vivo examination of glioblastoma tumours in laboratory mice. The approach was able to classify malignant and healthy brain tissue with up to 97% accuracy. These preliminary results represent an additional step toward eventual clinical use.
In research published in Neurophotonics, principal investigator Riccardo Cicchi, of the National Institute of Optics (CNR-INO) and the European Laboratory for Non-Linear Spectroscopy, and co-authors (also at the National Enterprise for nanoScience and nanoTechnology) conducted a study to discriminate healthy brain from glioblastoma tissues through the combination of Raman and reflectance spectroscopies. They used label-guided fluorescence to detect areas of tumour, followed by Raman and reflectance spectroscopies to examine and classify healthy and diseased tissues based only on their intrinsic molecular composition and oxygenation.
Laser-induced fluorescence spectroscopy has been widely used to discriminate malignant from healthy tissues. The researchers believe that diffuse reflectance and Raman spectroscopies could provide supplemental information. Diffuse reflectance is based on the ratiometric measurement of the spectrum of light backscattered by the tissue compared with the illumination spectrum. The measured spectrum characterizes tissue chromophores and scatterers related to the physiologic conditions of the tissue under investigation. Raman spectroscopy, based on inelastic scattering processes, generates a vibrational spectrum that’s characteristic of specific molecules in the imaged sample.
In vivo investigation
For the study, the researchers implanted EGFP-GL261 tumour cells into the brains of eight laboratory mice. EGFP-GL261 is a murine glioma cell line similar to human glioblastoma that expresses enhanced green fluorescent protein (EGFP). A cranial window provided optical access to the implantation site, while a second window placed nearby provided access to healthy brain tissue.
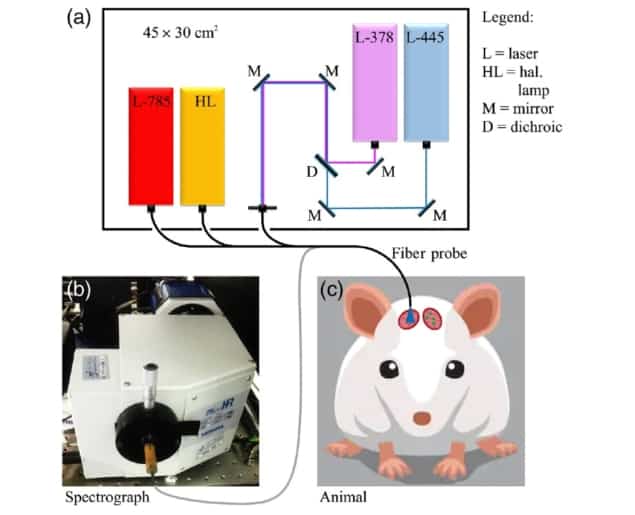
The researchers used fluorescence spectroscopy to localize cancerous areas in real time, by detecting the EGFP emission peak from 378 and 445 nm excitation. After placing an optical fibre probe at the point of maximum EGFP intensity, they then acquired fluorescence, Raman and reflectance spectra from the same region, and also performed spectral measurements on the healthy control tissue. They also developed a classification algorithm based on principal component analysis and linear discriminant analysis to discriminate the two tissue types based on their spectral profiles.
The tissue classification accuracies of Raman spectra and reflectance spectra were 92% and 93%, respectively. Combining both techniques improved the discrimination accuracy between healthy and tumour tissues up to 97%, due to the complementary information provided by the two techniques.
“Surgical resection plays a major role in current glioblastoma treatment, hence improving the detection of brain tumour areas in a fast and reliable way is key to a positive prognosis,” the authors write. “Our findings suggest that these spectroscopic techniques are able to recognize the altered metabolism of mouse glioma and its hypoxic environment.”
The researchers suggest that further investigations of brain tissues should also involve measuring absolute absorption, because tumours typically stimulate the formation of new blood vessels. They add that their successful use of EGFP labelling to precisely localize murine glioblastoma areas for recording Raman signatures could be a beneficial approach for other oncologic studies based on animal models.
With respect to use in humans, lead author Enrico Baria thinks that optical spectroscopy will be used to complement MRI. “A preoperative MR image will tell a surgeon where the tumour is grossly located,” he explains. “Then, a fibre-optic probe combining multiple spectroscopic techniques will be used for quasi-real-time detection of diseased areas, or – at least – for obtaining a quick diagnosis of suspicious tissues before their excision.”
“Optical spectroscopy will be used also for obtaining a fast diagnosis of the excised biopsies, in order to complement the results from standard intraoperative histopathology. In the far future, once the instrumentation and the analysis have been significantly improved, maybe optical spectroscopy will be used alone for some of these tasks,” Baria tells Physics World.



