CT is an integral part of adaptive image-guided proton therapy (IGPT). It is used to monitor changes in a cancer patient’s anatomy caused by weight loss and/or tumour shrinkage, as well as for treatment plan adaption. CT simulation scans are usually performed in imaging suites outside proton therapy treatment rooms. This set-up, however, can cause workflow inefficiencies and inconvenience for both staff and patients alike.
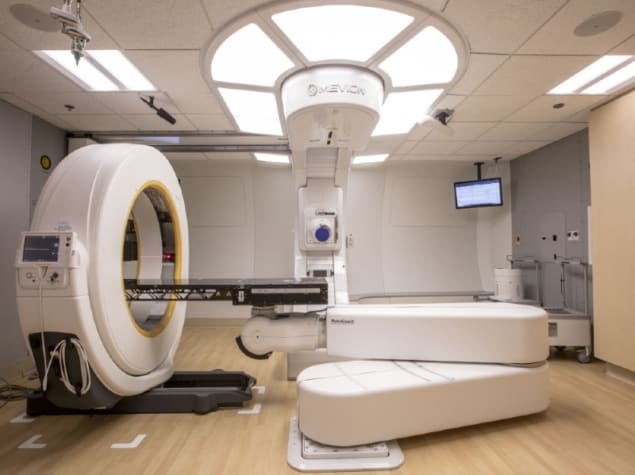
Compact mobile CT systems may change all this, by enabling scanning to be performed within proton treatment rooms. The Center for Proton Therapy at Orlando Health UF Health Cancer Center has successfully installed a mobile CT system for patient localization and set-up in its compact proton therapy vault. The research team has now described the commissioning process and the dosimetric implications of adaptive planning with the mobile system (J. Appl. Clin. Med. Phys. doi: 10.1002/acm2.12319).
The scanner (the AIRO Mobile CT System) being commissioned for use with the centre’s Mevion S250 proton therapy system is a 16-slice helical scanner that acquires images with 120 kV, 10-250 mA and a field-of-view (FOV) up to 51.2 cm. Designed for intraoperative surgery, the large FOV enables the scanner to capture the entire patient surface including immobilization devices and the treatment couch.
The commissioning process
A critical part of the commissioning process is setting up the stopping power curve for an in-room CT scan so that dose calculations on the scanner are dosimetrically matched to the treatment planning system.
“This groundwork is important so that if adaptive planning is performed on the in-room CT, one can be certain that the changes in dose are due to changes in anatomy and not to differences in the CT scanner or scanning protocols,” medical physicist Twyla Willoughby told Physics World. “This is very important in being able to make clinical decisions regarding adapting a treatment plan. When comparing two different CT scanners for dose comparison, any changes in CT values and in the calculated stopping powers can lead to changes in the dose along the proton path or to a change in the range of proton therapy.”
To do this, Willoughby and colleagues scanned an electron density CT phantom on a simulation CT scanner and the mobile CT, and compared the mean CT numbers to determine differences. They imaged a phantom containing 16 rods and 13 tissue substitute materials with varying plug patterns, table heights, and mA with fixed 120 kV. Images of plugs representing brain, lung 300, lung 450, cortical bone, adipose, breast, liver, solid water, and true water were analysed. They then determined the stopping power ratios (SPRs) by entering averaged CT numbers into a stoichiometric SPR calculation algorithm.
The last step of the commissioning process involved confirming dosimetric equivalence for dose calculated on CT scans from the two scanners. The researchers developed heterogeneous, single-field, non-robust plans on thorax, pelvis and head phantoms, to test the dose accuracy for proton beams traversing large areas of heterogenous media. They also generated five different clinically reasonable treatment plans on five different phantoms to test the accuracy of the adaptive system in common clinical scenarios.
Key findings
Lead author Jasmine Oliver and colleagues reported that proton dose calculations on CT image sets acquired by the mobile CT scanner could be used to calculate dose with relatively high accuracy, similar to the simulation scanner.
They cautioned that dosimetric equivalency testing, using visual display of isodose lines and water-equivalent thickness (WET) values between the planning and in‐room CT scanners, should be performed before any in-room CT system is deployed for adaptive planning purposes.
Test results showed that CT numbers differed between the scanners. Low-density plugs had a higher CT number in the mobile CT compared with the simulation scanner, while high-density plugs had a lower number. Dose on the mobile CT extended deeper by about 5 mm compared with the original treatment plan.
To create equivalent dose distributions, it was necessary to adjust the SPR curve’s low-density data points of the mobile CT, to obtain better proton beam range agreement based on isodose lines. When the authors compared the stochiometric-based SPR curve and the “dose-adjusted” SPR curve, they observed slight improvement on gamma analysis between the treatment plan and the mobile CT plan for single-field plans at the 1%, 1 mm level. Clinical plans at 3%, 3 mm demonstrated equivalent dose.
“Our results demonstrated that performing the stoichiometric analysis for a given phantom and CT scan may not provide dose equivalence between two different CT scans… it was important to verify the dosimetric equivalence of the two CT image sets with their corresponding stopping curves,” wrote the authors. “To achieve this, it was necessary to directly map CT values and adjust them to yield better dosimetric comparisons at the end-of-range.”
The mobile CT system in the proton treatment vault is currently used to perform “re-simulations” for patients who may have anatomical changes due to radiation therapy. “It is used on all of our breast patients to monitor target swelling, on lung patients to monitor fluid in the lungs and tumour changes, and on head-and-neck patients to monitor tumour shrinkage,” Willoughby explained. “These things dramatically affect the proton range and modulation, and can cause significant changes in the treatment plan if they go unmonitored.”
The cancer centre does not offer pencil beam scanning (PBS) proton therapy. However, the authors believe that, based on their experience, the image quality of the mobile CT scanner is good enough for dose-recalculation on PBS as well as double-scatter systems.



