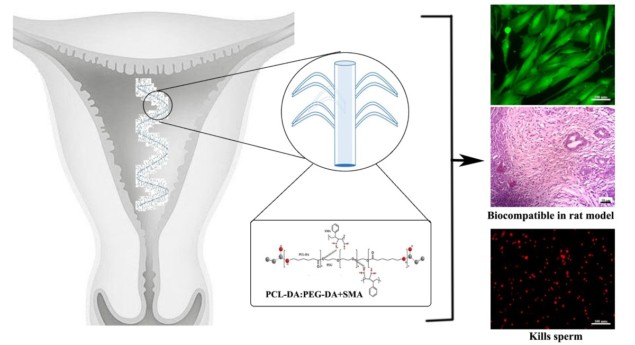
Researchers from India have developed a biodegradable gel for female contraception. Reporting their results in Materials Science & Engineering C, they found that the active ingredient styrene maleic anhydride (SMA) embedded in a biodegradable hydrogel kills sperm and prevents egg cell formation. Implanting the gel in female rats showed excellent bio-compatibility, paving the way for creation of a non-hormonal contraceptive implant. Such a gel would expand the hormonal and non-hormonal contraceptive options currently available for women, many of which cause side effects.
Potential for a non-hormonal female contraceptive
SMA is already in Phase III clinical trials as a male contraceptive implant in the vas deferens, the vessel transporting sperm in men. But in addition to killing sperm, SMA also disrupts the development of female egg cells. The astonishing combination of these two effects led the research team headed by Piyali Basak and Sujoy Guha to design a female version of the gel.
To achieve long-term dosing, their team wanted to incorporate SMA into a gel that would be inserted in the uterus, the female reproductive organ that nurtures the developing foetus till birth. A suitable gel has to be safe for the body, biodegradable and allow the incorporation of SMA without inactivating the drug.
To create such a gel, polycaprolactone (PCL) proved to be a good starting point. It is commonly used for medical applications as a biodegradable polymer; however, it is not flexible enough for this application. To achieve the required flexibility, the researchers mixed PCL with PEG (polyethylene glycol) and polymerized them as diacrylates (DA). The polymers were fabricated into a PCL-DA:PEG-DA hydrogel, a water-based gel made from an insoluble polymer. They then added five different concentrations of SMA and characterized the resulting gels.
Selecting the best concentration
First author Bhuvaneshwaran Subramanian and his team tested the physical properties and biodegradability of the gels, as well as the influence of exposure to ethanol, varying temperature and mechanical stress.

Out of the five samples with different SMA concentrations, the team further evaluated the three most promising ones using sperm and rat uterine cells. As intended, the gel killed sperm but did not harm the uterine cells. The sample with the highest spermicidal activity was then chosen for implantation in a rat.
Because rats do not have a true uterus, the researchers implanted the hydrogel in the fallopian tubes, which serve as a model for the human uterus. The implanted gel had no effect on any of the tested tissues or organs and degraded after 150 days. Blood analysis revealed no signs of inflammation, toxicological symptoms or metabolic and hormonal changes.
These findings suggest that the SMA hydrogel should be safe for implantation in the female reproductive organs. The next step will be to investigate whether the combination of SMA and the PCL-DA:PEG-DA hydrogel also works as an effective contraceptive when implanted in rats.