Lithium might be a "simple metal" under normal conditions but under extreme pressures it becomes a superconductor with one of the highest critical temperatures of all the elements
At ambient conditions the conduction electrons in lithium the first metal in the periodic table are only weakly perturbed by its atomic cores, which are arranged in a highly symmetric body-centred cubic lattice. For many years it was thought that lithium would remain metallic at high pressures, retaining some form of highly symmetric cubic structure. However, in 1999 Jeffrey Neaton and Neil Ashcroft of Cornell University predicted that lithium would undergo various structural phase transitions as the pressure was increased, leading to a “paired atom” or molecular phase with low symmetry. Moreover, the electronic properties of this phase are expected to be close to those of an insulator.
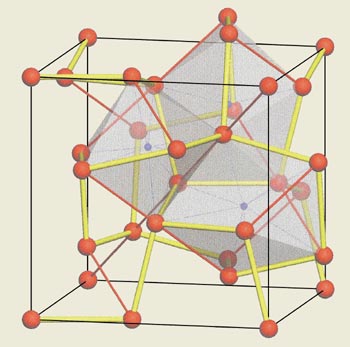
A year later X-ray diffraction experiments by the current author and co-workers showed that at pressures near 40 GPa (400 000 atmospheres) lithium transforms from a face-centred cubic phase, via an intermediate rhombohedral phase, to a cubic polymorph with 16 atoms per unit cell called cI16 (see figure). This structure can be viewed as a 2 x 2 x 2 super cell of a body-centred cubic structure but with the atoms displaced diagonally.
Calculations of total energy show that this structure is stable with respect to all the known structures for metals to pressures of at least 165 GPa. Moreover, the decreased symmetry caused by the diagonal displacement of the atoms changes the Fermi energy in a way that means lithium is no longer a simple metal. Although this cI16 structure was different from the “paired atom” structure proposed by Neaton and Ashcroft, it provided further evidence that lithium adopted low-symmetry structures at high pressure.
Experiments under pressure
The existence of low-symmetry phases at high pressure immediately poses the question of superconductivity in lithium. The only known superconducting alkali metal is caesium, which becomes superconducting at 12.5 GPa with a critical temperature of about 1.5 K. At this pressure it transforms into a low-symmetry orthorhombic phase with 16 atoms per unit cell. Transitions of the heavy alkali metals (caesium, rubidium and potassium) to low-symmetry structures are driven by electronic transitions in which delocalized s-valence electrons become more localized d-valence electrons. Similar transitions in lithium and sodium are driven by transitions in which s-electrons become p-electrons.
This possibility of pressure-induced superconductivity in lithium was initially studied theoretically. In 2001 Niels Christensen and Dmitri Novikov predicted that lithium would become superconducting at high pressures and that the critical temperature would reach 50-70 K in the region of the face-centred cubic to cI16 transition, and could be as high as 60-80 K in the cI16 phase.
Two experimental groups have now observed superconductivity in lithium at high pressures, albeit with lower critical temperatures than predicted by theory. Both groups used so-called diamond-anvil cells to compress the lithium.
Katsuya Shimizu of Osaka University in Japan and co-workers measured the electrical resistance of lithium as a function of temperature and observed a sudden drop in the resistance when the sample was cooled below 7 K at a pressure of 30 GPa (K Shimizu et al. 2002 Nature 419 597). The researchers attribute this drop in resistance to a transition to a superconducting state. Moreover, this transition temperature increases with pressure to reach a maximum of 20 K at 48 GPa. The Japanese team also demonstrated that the effect is suppressed by a magnetic field and disappears completely for fields above 3 T. This is generally considered as strong experimental evidence for superconductivity.
Shortly afterwards these results were confirmed by a team at the Carnegie Institute of Washington in the US that studied both the electrical resistance and the magnetic susceptibility, which is considered the ultimate evidence for superconductivity (V V Struzhkin et al. 2002 Science 298 1213). The critical temperatures from both methods were in good agreement.
The Carnegie researchers found that the critical temperature increased from 9 K at 23 GPa to about 15 K at 36 GPa, and they attribute this to the face-centred cubic structure. The critical temperature remained constant at about 16 K for pressures between 40 and 70 GPa, which they suggest is due to the cI16 phase.
The results of the Osaka and Carnegie experiments are in good agreement and the small differences between them are probably due to way the lithium was confined in the diamond-anvil cell. Lithium is very difficult to study at high pressure because it reacts with both the diamond anvils and the gasket material surrounding the sample. Isolating the electrodes that are used to measure the resistance from the gasket, which is normally made of metal, makes the experiments even more complicated. While the Osaka group confined the lithium sample in a small pit drilled in one of the diamond anvils with a laser, the Carnegie team used a metallic but nonmagnetic gasket for its susceptibility measurements.
The results also suggest that a sudden drop in the electrical resistance of lithium observed in an experiment at the University of California at Los Angeles in 1986 was due to a superconducting phase transition. Tzer-Hso Lin and Keh-Jim Dunn observed the drop at a temperature of 7 K and pressures between 22 and 32 GPa.
Theory under pressure
Lithium brings the number of elements that superconduct under pressure to 23, which is close to the number of elements that are superconductors at ambient pressure (29). Among the high-pressure superconductors are such unlikely candidates as oxygen and sulphur, and even iron becomes superconducting in a small pressure range. However, the maximum critical temperature observed in lithium (between 16 and 20 K) is among the highest observed for any element, and attention will now turn to the other alkali metals.
Rubidium and potassium have the same low-symmetry structure as caesium, only at higher pressures, while sodium follows a sequence of phases that is similar to lithium, again at higher pressures. The discovery of superconductivity in lithium and its explanation will also have important implications for superconductivity in highly compressed metallic hydrogen. Hydrogen is the only element in the first group of the periodic table that is not a metal at ambient pressure (see Physics World July 1995 pp4347).
The discoveries of low-symmetry phases and superconductivity in lithium are good examples of the intensive interactions between theory and experiment in modern high-pressure physics. Theoretical predictions stimulate experimental studies, which result in the need for more theoretical work, which in turn leads to further experimental measurements. However, the agreement between theory and experiment is not always perfect. The “paired atom” phase has not been observed experimentally and the critical temperatures measured by the Osaka and Carnegie teams are lower than the theoretical predictions by a factor of three or four. The challenge is now on for the theorists to refine their calculations and explain the latest experimental results.