Exotic, ultracold states of matter are challenging physicists to draw on expertise from atomic, condensed-matter and plasma physics, and causing a few surprises along the way

The ultracold world has fascinated and surprised scientists since 1911, when Heike Kamerlingh Onnes discovered superconductivity in mercury at 4.2 K. Now physicists routinely achieve temperatures millions of times colder.
When atoms are cooled this close to absolute zero, they fall into the lowest possible quantum state with bizarre consequences. For example, Bose-Einstein condensates, created for the first time in 1995, are systems where the atoms have undergone a collective identity crisis and fallen together into the same quantum state. Fermi gases, on the other hand, are prevented from this collapse by basic quantum-mechanical principles, and become incompressible. The creation and understanding of Bose-Einstein condensates and Fermi gases represent major advances in ultracold atomic physics (see Physics World August 1999 pp37-42 and April 2002 pp27-31).
But now there is a completely new ultracold system to explore ultracold neutral plasmas. These systems bridge the gap between atomic physics and plasma physics, and between plasma physics and condensed-matter physics. In addition to being fascinating in themselves, these exotic and relatively unexplored states of matter may also help us to understand the surfaces of neutron stars and the centres of planets such as Jupiter.
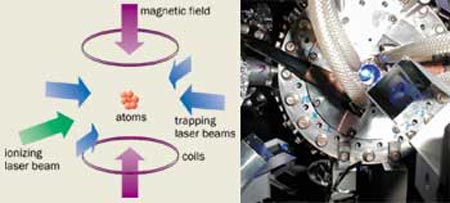
Plasmas are forms of matter in which a significant fraction of the neutral atoms and molecules have been ionized to form free electrons and ions. Ionization usually results from energetic collisions between particles, which means that most plasmas such as the surface of the Sun or a fluorescent light bulb are relatively hot. But it is also possible to create ultracold plasmas, by using lasers to trap and cool neutral atoms to temperatures of 1 mK or lower. Another laser then ionizes the atoms by giving each of the outermost electrons just enough energy to escape the electrical attraction of its parent ion (figure 1).
The key point about ultracold plasmas is that by manipulating the atoms with lasers, the kinetic energy of the liberated electrons can be controlled. Using standard pulsed lasers, the electron energy can be made to correspond to a temperature of as low as 0.1 K a limit set by the frequency bandwidth of the laser pulse. The ions, however, retain the millikelvin temperatures of the neutral atoms. This type of non-equilibrium ultracold plasma evolves rapidly, and many fundamental questions about its behaviour remain unanswered. Experiments conducted so far have revealed surprising dynamics and recombination behaviour that are pushing the limits of our knowledge of plasma physics.
A subtle change of experimental procedure can produce a system that is closely related to ultracold plasmas a cold, dense Rydberg gas. Tuning the laser wavelength slightly below the ionization energy leaves the atoms in highly excited Rydberg states in which the outer electron has a large radius. Compared with atoms in the ground state, these large “floppy” atoms have exaggerated properties, and are easily influenced by their environment. The more highly excited the atoms are, the more susceptible they are to environmental conditions, and the stronger their interactions with each other. Rydberg atoms do not move or collide because they are laser cooled, but the electron orbits of adjacent atoms can overlap. This leads to a bizarre state of the system that blurs the distinction between a plasma and a collection of neutral atoms.
Investigating and understanding the properties of ultracold plasmas and Rydberg gases requires a combination of experimental, theoretical and computational techniques from a variety of different subdisciplines in physics.
Making an ultracold plasma
The story of ultracold plasmas began a few years ago at the National Institute of Standards and Technology (NIST) in Gaithersburg, Maryland. We had been working with laser-cooled metastable xenon atoms and wondered what would happen if we could ionize the entire sample in an instant. For several years prior to our work, the laser-cooling group at NIST had been studying cold atom collisions, some of which produced ions. Work on ultracold plasmas seemed to be a natural extension of this, but instead of making ions by the handful, a million could be made in an instant. We were also motivated by the efforts to create antihydrogen at CERN. One approach to making antihydrogen begins by holding cold positrons and cold antiprotons in the same electrical trap. In this system it may be possible to use a laser to stimulate recombination of the positrons and antiprotons to encourage antihydrogen formation. We naively thought that by ionizing the ultracold atoms in our trap, we would be running the CERN process in reverse. Optimizing the laser-recombination method with a simple system like ours might therefore help guide a similar experiment for antihydrogen.
Another intriguing aspect of the ultracold-plasma experiment was that it seemed we could create a system in which the initial conditions were completely under our control. By choosing the initial density and temperatures of the ions and electrons, it would be possible to arbitrarily set the interaction strength between the atoms and ions in the plasma. Neutral atoms in the ground state collide like billiard balls and the atomatom interaction is very weak, especially at low temperatures. However, the interaction between colliding Rydberg atoms is much stronger and this increases with the Rydberg excitation because the “orbiting” electron is so weakly attached to its parent ion. When atoms are ionized to form a plasma in which the liberated electrons have essentially zero kinetic energy, the interactions between the ions are very strong. Indeed, the ions are almost always in collision due to the long-range nature of the electrical force, and as the kinetic energy of the particles in the plasma increases, the interactions become weaker.
The ionization step needed to create an ultracold plasma is performed using nanosecond laser pulses. The kinetic energy of the liberated electrons is essentially the difference between the laser’s photon energy and the ionization energy. The lowest electron energy achievable with our pulsed laser corresponds to a temperature of about 0.1 K. It is straightforward to ionize as many as 50% of the trapped atoms, so the peak plasma density in our xenon experiments was something like 1010 per cubic centimetre.
Electrons with a temperature of T = 0.1 K travel at about 100 m s-1 so you might expect them to speed away from the sluggish ions within a few microseconds, leaving a positive core. But as a net positive charge develops near the centre of the plasma, Coulomb attraction pulls the electrons back. The ions spontaneously form a trap, and, depending on the experimental conditions, more than 90% of the electrons cannot escape the pull.
During this time the electrons thermalize and settle into a spatial distribution that closely follows the density of the ions in the central region of the ion cloud. This is the essence of so-called Debye screening in a plasma: wherever the electron and ion densities differ, electric fields develop that push the system back towards neutrality. Of course, there is a small net shortage of electrons and this results in an outer shell of positive charge. To date, experiments have probed only the central, nearly neutral portion of the plasma because the available diagnostics can only determine the plasma’s average properties.
Once the electrons have thermalized, the ions are initially frozen in place because of their relatively large mass and low temperature. The electrons race back and forth across the ion cloud, rebounding off the Coulomb potential and transferring momentum to the ions. The electrons exert a pressure on the ions just like an ideal gas exerts pressure on the walls of a confining box. This gets the ions moving. The electrons follow and the entire plasma starts to expand. The initial ion cloud has a Gaussian density profile, just like the neutral trapped atoms from which the plasma is made. Due to the dependence of the pressure on the density of the electrons, the plasma maintains its Gaussian shape and its characteristic size doubles in about 10 µs. In hindsight, this picture of the expansion is quite straightforward, but initially it was puzzling because the electrons appeared to be transferring energy to the ions without heating them very much, instead causing them to move outwards. This is different than the random thermal motion normally meant by “heating”.
The collisional thermalization between electrons and ions is very slow, due to the large difference in their masses, and it occurs on a millisecond timescale. The kinetic energy of the outward-moving ions in temperature units may be of the order 100kB, where kB is Boltzmann’s constant. However, a true measure of the ion temperature the deviation of an ion’s velocity from the local average velocity should remain much colder.
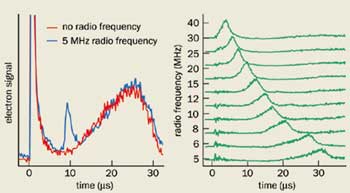
Measuring the outward motion of the ions, at least indirectly, is easy. Oscillating electric fields applied by grids above and below the plasma can resonantly excite its electrons. The response of the electrons is strongest when the frequency of the driving field resonates with the plasma frequency, which is governed by the physical properties of the plasma and scales with the square root of the electron density. For ultracold plasmas this frequency is typically between 1 and 300 MHz. At resonance, the electrons gain energy, which increases the rate at which they escape from the Coulomb well formed by the ions (figure 2. This rate is monitored by detecting the escaping electrons with an electron multiplier, allowing us to determine the average density of the plasma as a function of time. With this information the size and expansion rate of the plasma can be inferred.
Extraordinary expansion
Under most conditions, the kinetic energy of the plasma expansion is directly proportional to the initial energy given to the electrons during the ionization step. However, when the electron energy is decreased so that the kinetic energy is comparable to the Coulomb potential energy between neighbouring charged particles, the plasma expands much faster than expected. Determining the source of this extra energy has inspired theoretical studies by Stepahne Mazevet, Lee Collins, James Hanson and their colleagues at Los Alamos National Laboratory; Francis Robicheaux and his group at Auburn University; and Tom O’ Neil and colleagues at the University of California in San Diego. In all simulations, they observed a heating of the electrons during the first fraction of a microsecond, which agrees with the experimental results. However, there is still some disagreement as to the exact source of the heat.
The heat may come from electronion recombination. Electrons lose energy when they combine with an ion, and that energy is transferred to the remaining free electrons. It may also come from the electrical potential energy of the initial system. The neutral atoms from which the plasma is created are randomly distributed in a gas, which means the ions and electrons can have a small initial kinetic energy but a very large electrical potential energy. An additional source of heating may come from “continuum lowering”, in which neighbouring charged particles influence the ionization energy and cause the escaping electrons to have more energy than we expect. All of these factors probably contribute to the plasma expansion to some degree, and sorting out these contributions is one of the issues being addressed by theorists in this field.
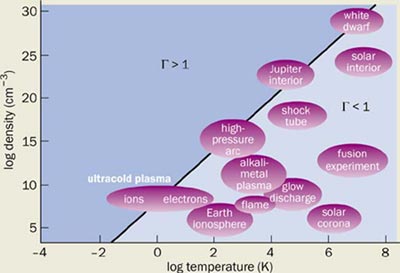
It is the understanding of these expansion dynamics that is exciting, because it may be that ultracold plasmas cross over to the regime of strongly coupled plasma physics. Plasmas are strongly coupled when the ratio of the Coulomb potential energy between neighbouring charged particles and the thermal kinetic energy, Τ = e2/4πε 0akBT, becomes greater than 1 (here a is inter-particle spacing, e is the electron charge and ε0 is the permittivity of free space). Traditional plasma physics confines itself to the regime where Τ figure 3).Basic concepts need to be revised in these systems, such as the complex processes of recombination, Debye screening (in which the different charges arrange themselves to minimize the overall electric field) and the hydrodynamic descriptions of plasmas.
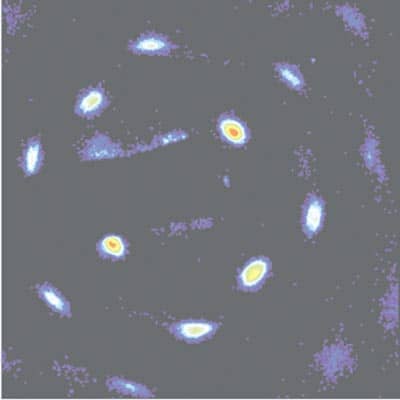
The recent progress in understanding these multicomponent plasmas containing ions and electrons has stimulated studies on single-component plasmas in which only electrons or only ions are trapped. Experiments by David Wineland’s group at NIST in Boulder, Colorado have raised some interesting questions. The researchers showed that as Τ increases, a cold non-neutral plasma changes from a gas-like to a liquid or even a solid structure as the particles develop spatial correlations to minimize the potential energy (figure 4). . Is it possible to create a two-component system in the laboratory with both electrons and ions strongly coupled? How would such a strongly coupled two-component system behave? Is a correlated two-component system a collection of neutral atoms, or is there some metastable state in which the positions of the ions and electrons are all correlated while the electrons are free to move from one ion core to another? The answers to these questions require concepts from condensed-matter, atomic and plasma physics, which is one of the most exciting aspects of ultracold-plasma research.
Dense Rydberg gases
There were big surprises early in our exploration of ultracold neutral plasmas. To make the electrons as cold as possible, we had to calibrate the laser wavelength so that it gave the electrons just enough energy to separate from their parent ions and no more. The obvious way to find that ionization energy was to simply shine the laser on the atoms and look for ions. We could then gradually reduce the photon energy until the ion signal disappeared. But the ion signal did not disappear. Below the ionization energy, when the atoms were excited to Rydberg states, we found ions galore. The system of ultracold Rydberg atoms left on its own had somehow spontaneously ionized.
This observation was reminiscent of work on room-temperature cesium atoms in the early 1980s by Serge Haroche’s group in Paris. In these experiments, two Rydberg atoms collided to form an ion, a free electron and a less excited neutral atom. The free electron went on to collide with several other Rydberg atoms, ionizing them and producing more electrons. If the density of Rydberg atoms was high enough for two of them to collide, the free electron from that collision would trigger an avalanche that ionized the rest of the Rydberg atoms.
However, our observations of ultracold xenon atoms did not quite fit this model. The atoms in our experiment were moving at about 6 cm s-1, which meant that no Rydberg atoms could collide for the first few hundred microseconds. But we observed spontaneous ionization much quicker than that. In addition, the free electrons coming from this spontaneously ionized Rydberg gas were moving more slowly than the free electrons from a photoionized sample. It was difficult to know if this was a consequence of the process of electron formation, or if it was due to some other effect in the plasma, such as Debye shielding.
The exact cause of spontaneous ionization in a Rydberg system is still uncertain, and while several prominent researchers are narrowing down the possibilities, the subject is somewhat controversial. Tom Gallagher at the University of Virginia has recently gathered evidence that suggests the spontaneous ionization of the Rydberg atoms is due to the same mechanism identified by Haroche. Using a modest radio-frequency field, Gallagher heated the electrons once they were formed, which dramatically increased the rate at which the avalanche proceeded. The spontaneous ionization of the Rydberg system requires energy from somewhere, and collisions between Rydberg atom followed by avalanche ionization seem to provide that energy. Perhaps the first few electrons are created when two Rydberg atoms are “born” in a collision.
Ed Eyler and Phil Gould at the University of Connecticut have suggested that the Rydberg-atom collisions that cause the ionization are not necessarily the result of billiard-ball-type collisions. The van der Waals forces between highly excited Rydberg atoms are strong, causing them to naturally attract one another at long range. The Rydberg atoms perhaps form long-range molecules and collide due to the molecular potential. The collisions would be violent enough that at least one of the Rydberg atoms became ionized.
Another possibility has been put forward by George Raithel at the University of Michigan. Raithel’s work suggests that the first few free electrons are not produced by Rydberg atom collisions but when Rydberg atoms are photoionized by black-body radiation. This broad-spectrum radiation is emitted by the rest of the experiment, which is at room temperature. When the free electrons collide with the Rydberg atoms they scramble the initial Rydberg state. This prevents any radiative decay of the atoms and encourages black-body and collisional ionization.
Harnessing floppy atoms
If the source of the first electrons could be pinned down, it might be possible to create an experiment that prevents the ionization. It is the first few free electrons that apparently cause the avalanche ionization of the rest of the system. Therefore, if these electrons are generated by overlapping Rydberg atoms in the initial gas, the atoms could be put in an optical lattice and kept apart. Michael Noel at Bryn Mawr College in Pennsylvania is creating an experiment to examine this possibility. If the idea is right, it may be possible to drive an insulatormetal transition in a high-density Rydberg gas.
In 1936 Nevill Mott made his famous study of the insulatormetal transition, commonly called the Mott transition. Certain compounds are insulators at room temperature but conductors at lower temperatures. Mott imagined that atoms in the insulator were regularly spaced on a 3D lattice. He calculated that if the distance between the atoms in the lattice changed enough, by changing the temperature for example, the electron clouds from neighbouring atoms would overlap. The electrons would then be free to wander from one lattice site to another the solid would become metallic.
In the Rydberg system, instead of shrinking the lattice constant to make the atoms overlap, you can simply increase the size of the atom by increasing its excitation. But contrary to atoms in solid-state systems, lower energy states are available in Rydberg atoms. When gas atoms in Rydberg states begin to overlap, one electron may be sent to a lower energy state while the other one takes away the excess energy. The Mott transition in an excited-state gas-phase sample therefore remains to be understood.
On the other hand, if the first free electrons come from black-body radiation, then the obvious solution is to get rid of that radiation. Raithel is building a cryogenic system that should eliminate black-body radiation. If, however, the free electrons come from exaggerated van der Waals forces in Rydberg atoms, then nothing can be done to prevent the massive avalanche ionization of the system.
Cold, dense Rydberg gases continue to generate challenging opportunities at the confluence of atomic and condensed-matter physics. This system exists at the quantumclassical interface, where calculations are difficult because it is not described by either one regime or the other. It also blurs the distinction between single-particle interactions and many-body interactions. Aside from possible technical applications, studies of this system will help us understand how to think about these complex issues at a fundamental level.
The latest challenge
The next generation of ultracold-plasma experiments due to begin this year will open up new possibilities. The experiments, which are being built in laboratories at Rice University and at Brigham Young University, use the elements strontium and calcium. These group-II atoms have the distinct advantage that they still have one loosely bound electron when they are ionized. This means that the ions have transition frequencies that can easily be driven by currently available lasers, and tools that are used for laser cooling and atom manipulation may also be used on the plasma.
A technique known as absorptive imaging the workhorse of experiments on ultracold neutral atoms will also be directly transferable to strontium and calcium. This produces a directly observable image of the spatially varying density profile of the plasma. Measuring these density profiles is essential to discriminate among the theoretical simulations of how the plasma density changes with time. Some models predict a shock wave developing during the plasma expansion, while others show ion acoustic waves freezing into place.
By using lasers to cool the ions in the plasma, it will also be possible to reduce the kinetic energy of the ions so that they are strongly coupled. In experiments like these it will be possible to verify and extend the theory of strongly coupled two-component systems. In particular, we will be able to study recombination, collision dynamics, electron heating and expansion in a negative-pressure system.
Ultracold neutral plasmas and dense Rydberg gases are close cousins in this new field of ultracold, highly excited, strongly interacting atomic systems. They inhabit the regions between atomic, plasma and condensed-matter physics. They have already surprised us with puzzling questions some of which we have started to address but every new experiment asks more questions than it answers.