Joost van Mameren explains how quantitative force measurements by optical tweezers can unravel the mechanical properties of biological molecules
First demonstrated over 20 years ago, optical tweezers have become an established tool in research fields ranging from biophysics to cell biology. As their name suggests, optical tweezers use beams of light to hold and manipulate microscopically small objects such as biological molecules or even living cells. They are formed when a laser beam is tightly focussed to a tiny region in space using a microscope objective as a lens. This region becomes an optical trap that can hold small objects in 3D.
Optical tweezers can also make accurate measurements of the tiny, sub-picoNewton forces exerted on the trapped objects. This allows researchers to study the diffusion dynamics (or Brownian motion) of an object in a solvent — a property that can play a key role in the function of many biological molecules. Optical tweezers can also be used to micromanipulate an object using well-controlled forces.
Trapped by refraction
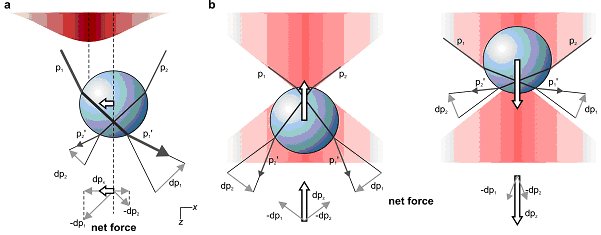
The trapping force that holds an object in place in optical tweezers can be understood by considering how the object refracts light. Because it is tightly focused, the laser light is most intense at the centre of the trap, which means that if the object moves slightly away from the centre in a transverse direction, one part of the object will refract less light than the other. As a result, the object refracts more light away from the centre of the trap than towards it. Light carries momentum and the net effect of this refraction is a force that deflects some of this momentum away from the centre of the trap. By Newton’s third law an equal and opposite force must act on the object, pushing it towards the centre of the trap (see figure 1a).
A similar refraction-related effect also causes the object to push back in the opposite direction of the laser beam (see figure 1b). The trapping is stable only if the force of the laser light scattering from the particle along the positive z–direction is compensated by a trapping force along the negative z-direction. To achieve this, a very tight focus is needed, with a significant fraction of the incident light coming in from large angles. This can be achieved using a lens with high numerical aperture.
Force-sensing optical tweezers have the additional ability to track the motion of an object within the trap — information that can then be used to calculate any external forces acting on the object. External forces — notably the Brownian forces caused by an object being continuously bombarded by solvent molecules — tend to displace the object from the centre of the trap. Using interferometry measurements of the light refracted from the object, this displacement can be determined to nanometre accuracy, which allows the external forces to be measured at the sub-picoNewton level.
Such external forces depend on the viscosity on the solvent and the properties of the trapped object. In addition, the trapped object can be pushed or pulled on other objects and the forces involved can be measured.
Since their invention, optical tweezers have been used with great success in the field of single-molecule biophysics. For example, they have helped researchers unravel the complex elasticity and folding dynamics of DNA, RNA, proteins and other long-chain “biopolymers”. In these experiments, the biopolymers are typically manipulated from both ends either by suspending them between an optical trap and a surface, or between multiple traps. Data obtained using optical tweezers complement measurements made using other techniques for measuring the forces on single molecules – including atomic-force microscopy (AFM).
Optical tweezers have also helped further our understanding of how “motor proteins” such as kinesin and myosin convert chemical energy into work. Such biological motors operate over distances of nanometres and with picoNewton forces — and the desire to understand motor proteins has been an important driver in the development of force-sensing optical tweezers technology.

Indeed, a plethora of mechanically active enzymes have been studied in this way, including many involved in DNA metabolism — biochemical processes involving DNA. While the number of studies in this field is increasing, there are still many biological and biophysical questions that could be addressed using optical tweezers, including the intricate mechanics of the replication, transcription and repair of DNA. While researchers are beginning to focus on the actions of individual enzymes during these processes, the coordinated action of the sometimes tens of proteins involved in real-life reactions remains largely enigmatic.
Optical tweezers have also been used to study living biological cells. Initially, they were used mainly to sort, manipulate, push and pull cells in a qualitative manner. However in the last year or so, optical tweezers have been used to make quantitative measurements in or around live cells. For example, they have been used to study the mechanics of phagocytosis — the process by which a cell engulfs and ingests foreign particles.
Off-the-shelf system
Such measurements were once the sole domain of customized research tools, but recently JPK Instruments of Germany introduced its NanoTracker experimental platform. The first off-the-shelf quantitative optical-tweezers system, the platform is designed for experiments in the life sciences and can trap and measure the motion of particles ranging in size from microns down to a few tens of nanometres.
This compact system is based on a commercial inverted optical microscope, such as the Axio Observer from Carl Zeiss (see figure 2). The optical layout of the NanoTracker is schematically depicted in figure 3.
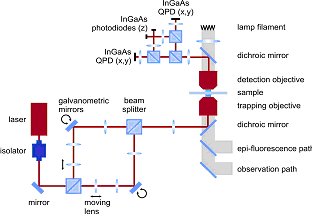
Two optical traps are created using a laser steering unit comprising two individual beam paths that are separated by polarization and optimized for stability. The customized microscope body features both a long-distance motorized XY translation stage and a closed-loop 100×100×100 µm3 piezo stage.
A photodiode-based detection unit is positioned on top of the microscope to detect the 3D forces and displacements for the two traps (see figure 3). To increase sensitivity, separate detectors are implemented for lateral (XY) and axial (Z) detection. Moreover, axial detection is further enhanced by the use of an aperture stop to reject high-angle rays.
Although the platform is designed to meet the needs of scientists who are pushing the boundaries of single-molecule biophysics, it is also versatile, user-friendly and safe enough to be used by researchers not used to working with lasers and optics. As such, the NanoTracker covers a broad range of quantitative nanomanipulation needs. With the NanoTracker, optical tweezers have evolved from being a specialist research tool developed and used by experts, to an off-the-shelf system able to serve a much wider community of researchers in the life sciences.



