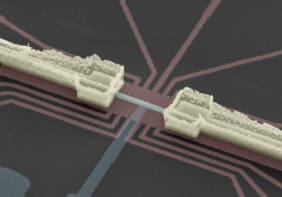
Physicists have succeeded in measuring the conductance of a single hydrogen molecule for the first time. Jan van Ruitenbeek of Leiden University in the Netherlands and co-workers used a mechanically controlled “break-junction” device to trap a single hydrogen molecule between two platinum electrodes and measure its conductance (RHM Smit et al. 2002 Nature 419 906). This “hydrogen bridge” represents a simple test system in which fundamental properties of single-molecule devices can be explored.
The field of molecular electronics has made steady progress in recent years and components made from single molecules have the potential to overcome the limits of silicon-based microelectronics. However, it is important to establish that single molecules, rather than the contacts, are responsible for the phenomenon observed in experiments.
Van Ruitenbeek and co-workers showed that a single hydrogen molecule can form a stable bridge between two platinum electrodes. Moreover, the bridge was found to have a conductance that was close to the value of the fundamental quantum unit of conductance, 2e2/h where e is the charge on the electron and h is Planck’s constant.
In the break-junction technique a knife is used to make an incision in a macroscopic metal wire which is then mounted inside a vacuum container and cooled to 4.2K. The sample wire is then bent, which causes it to break at the notch. The freshly exposed fracture surfaces are then brought back into contact. The separation of the two electrodes can be adjusted with a piezoelectric element.
“The importance of the experiment is that the system is simple and allows for detailed comparison with calculations,” van Ruitenbeek told PhysicsWeb. “Once we agree with theory on this simple model system, we have better hopes for understanding the more complicated molecular devices. It will get really interesting as soon as we come to molecules that have intrinsic diode characteristics or non-linear properties.”
The group now plans to look at the combination of hydrogen with different types of metal electrodes, including ferromagnetic and superconducting contacts.