Everyone has seen water droplets splash off a surface but this phenomenon is more complicated than it first appears, as recent experiments at the University of Chicago have demonstrated. Not surprisingly, the experiments show that the viscosity of the liquid plays a critical role, but so does the pressure and molecular weight of the gas that the drop falls through (L Xu et al. 2005 arXiv.org/abs/physics/0501149). The results could be relevant in applications such ink-jet printing and the combustion of liquid fuels.
Scientists have been interested in splashes since at least the late 19th century when A M Worthington photographed what happens when drops of milk or mercury hit a smooth surface. Harold Edgerton and colleagues also photographed drops hitting thin layers of fluid in the 1950s. In general, when a drop hits a solid surface it spreads out and breaks up, creating a splash of smaller droplets.
Sidney Nagel and colleagues at Chicago have now seen something that no one has seen before by releasing drops of alcohol from various heights onto a glass microscope slide inside a vacuum chamber and recording what happens with a high-speed video camera. The team used three liquids with different viscosities (methanol, ethanol and 2-propanol) and four gases with different molecular weights (helium, air, krypton and sulphur fluoride) inside the vacuum chamber. Moreover, they varied the pressure in the chamber from just 1 kilopascal up to 100 kilopascals (atmospheric pressure).
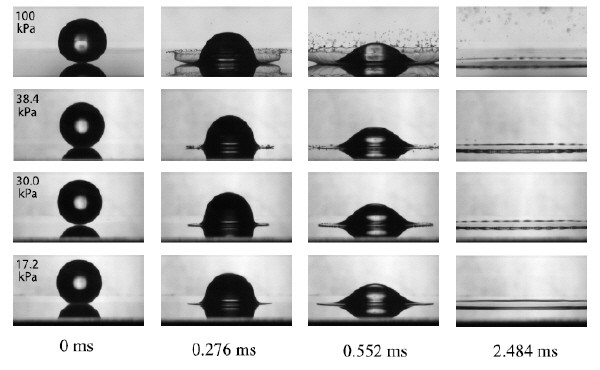
To their surprise, the Chicago physicists found that the surrounding gas played a key role in the splashing process. In particular, they found fewer droplets were ejected from the surface as the pressure was lowered, and that no droplets emerged below a threshold pressure (see figure). They also found that the threshold pressure scaled with the molecular weight of the surrounding gas. Moreover, they found that 2-propanol, which has the largest viscosity of the three liquids, had the lowest threshold pressure.