Researchers in the US have developed a new type of organic molecular transistor that can sense and respond to its chemical environment. The device, made by Colin Nuckolls at Columbia University and colleagues at the Brookhaven National Laboratory, consists of hydrocarbon molecules lying in the gap created when a single-walled carbon nanotube is cut in half. Since the electrical conductivity of the hydrocarbons changes signifcantly when the device is exposed to other molecules, the transistors can work as ultrasensitive chemical detectors (Proc. Natl. Acad. Sci. at press).
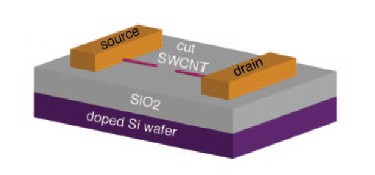
Nuckolls’ device consists of a silicon substrate on which a single-walled metallic carbon nanotube has been grown using chemical vapour deposition. The tube is then cut in half using an ultrafine lithographic technique, leaving a very small gap of 2 to 6 nm. Large metal pads are then attached to the other ends of the nanotube to act as the source and drain of the transistor, with the silicon surface acting as the gate. When a voltage is applied to the electrodes, a current flows between the source and drain (figure 1).
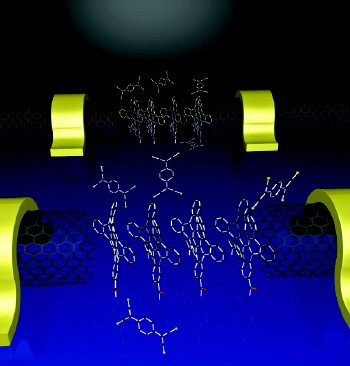
Nuckolls and co-workers then self-assembled a layer of polycyclic aromatic hydrocarbons, just one molecule thick, between the source and drain. The hydrocarbons arrange themselves in a line between the ends of the nanotube (figure 2). Since the electrical conductivity of the molecules depends on the local chemical environment, the device can be used as a sensor.
For example, when the device was exposed to electron-deficient molecules such as tetracyanoquinodimethane (TCNQ), the conductivity of the hydrocarbons was found to increase so much that the current passing through the transistor goes up by an order of magnitude. This produced a clear electrical signal that could be easily measured. Although the scientists are not entirely sure why the conductivity changes, they think the TCNQ probably acts a dopant by accepting π-electrons through charge transfer between the electron-deficient TCNQ and the electron-rich hydrocarbons. They also say the device could be used to detect chemicals in air or even be immersed in liquids.