Researchers in the US have made a new class of materials called "nanopolymers" -- the first nanoscale equivalents of polymers. The breakthrough was made by Francesco Stellacci and colleagues at the Massachusetts Institute of Technology and involves introducing defects onto two opposing areas on the surface of spherical-shaped metallic nanoparticles. The resulting divalent particles are then chained together to make freestanding films (Science 315 358).
Nanoparticles are nanometre-sized collections of atoms that can be used as building blocks to make a wide variety of materials, such as supercrystals or ionic liquids. However, they lack the ability to bond along specific directions — like atoms and molecules do — which means they are not easily joined together to make large structures like filaments or films. This is because nanoparticles are typically coated with a capping layer to prevent further growth or clustering.
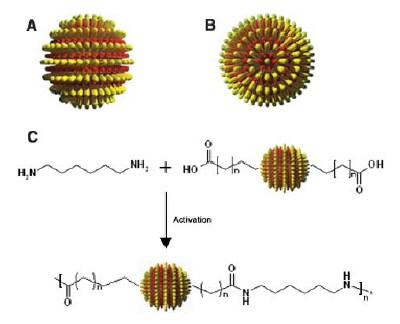
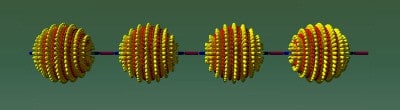
Now, Stellacci and colleagues have found a way to overcome this problem. The researchers effectively break the symmetry of the round nanoparticles by bonding two different types of ligand, such as thiol molecules, onto the poles of the spheres. The ligands on one nanoparticle are then free to bond with the ligands on the other particles so they can then be chained together to form the nanoscale equivalent of polymers (figures 1 & 2). The chaining reaction, which takes just a few hours, is very similar to way nylon polymerizes to form chains, says Stellacci.
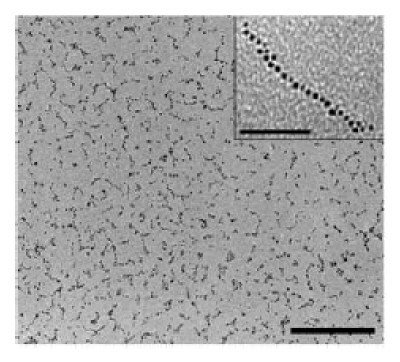
The scientists confirmed their result by taking tunnelling electron microscope images of the nanoparticle chains. The number of nanoparticles in each chain varies widely but the maximum number counted was 50,000 nanoparticles molecularly linked together (figure 3). Some chains even produced a continuous film as large as a square centimetre across and 60 µm thick.
“The main application of this work is in the generation of a new class of materials called nanopolymers with substantially novel properties, such as controlled porosity on the nanoscale,” said Stellacci. “These polymers will allow fundamental investigations of material properties – for example, can such materials retain glass transition temperatures and if so, how viscous is the glass?”
The team now plans to make longer chains of nanoparticles.