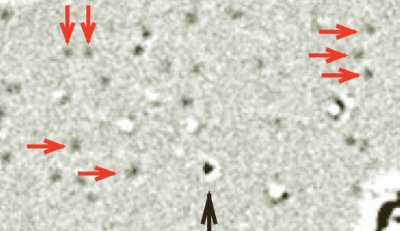
Physicists in the US claim to have used a transmission electron microscope (TEM) to see a single hydrogen atom – the first time that a TEM has been used to image such a light atom. The breakthrough was made by supporting the atom on graphene — a sheet of carbon just one atom thick. The team has also been able to watch hydrocarbon chains move across the graphene surface, suggesting that the technique could be used to study the dynamics of biological molecules.
There is nothing new in using TEMs to see individual atoms, but until now such instruments could only be used to image heavy atoms. One reason is that a TEM creates an image by shining an electron beam on a sample and measuring how much it is deflected by atoms of interest. Lighter atoms deflect electrons less than heavier atoms, which means that only the latter show up on an image.
Another problem is that a sample in a TEM has to be supported on a substrate that is durable enough not to be damaged by the electron beam, but thin enough for most of the electrons to pass straight through. Thin metal films or semiconductor foils are usually chosen as substrates, but these are still much thicker than single atoms and contain atoms heavier than carbon or hydrogen. Scattering from the substrate therefore tends to swamp the already weak signal from lighter atoms.
Thinnest and toughest
Now, however, Jannik Meyer, Alex Zettl and colleagues at the University of California, Berkeley have found away around this problem by using graphene, the thinnest and toughest known material, as a TEM substrate ( Nature 454 319 ).
The team came up with the idea while using a TEM to study defects in graphene. However, they also discovered that they could identify individual carbon and hydrogen atoms — as well as hydrocarbon chains — that had contaminated the surface of the graphene.
A crucial feature of the technique is that carbon atoms within the graphene lattice are invisible to the TEM — even though the technique can clearly see a single carbon atom on the surface of the graphene.
The carbon sheet provides a uniform background that shows no structure on its own Jannik Meyer, University of California
“The carbon atoms are packed in a regular arrangement with a spacing that is not resolved in this microscope,” explained Meyer, adding “thus, the carbon sheet provides a uniform background that shows no structure on its own”.
In addition to seeing individual atoms, the team was able to watch as the electron beam created the occasional hole in the graphene substrate. They even saw one such hole being repaired as the graphene absorbed carbon atoms from the surrounding environment.
Dynamical behaviour
The team was also able to study the dynamical behaviour of hydrocarbon chains (thought to be alkanes) that attached themselves to the graphene. These molecules were being imparted with energy by the electron beam, and so the researchers were able to watch them move around on the surface.
Zettl told physicsworld.com that the team is particularly interested in using the technique in the development of functionalized nanostructures — tiny objects that are engineered to perform a specific function. These are often hybrid materials — say a carbon nanotube decorated with biologically active molecules — and Zettl believes that TEM could be used to understand the real-time chemical binding or molecular dynamics processes that make such materials function.
“Furthermore, we are expanding our studies of the mechanical and electronic properties of graphene itself, and these TEM methods are expected be highly useful for that too,” added Zettl.
The team is also confident that others will use graphene substrates in their TEMs. “Chances are that these membranes will soon be used in TEM labs around the world,” said Meyer.
Contamination concerns
Debbie Stokes, an electron microscopist at the UK’s University of Cambridge agrees that graphene is a good substrate for supporting a TEM specimen, because it has minimal influence when imaging the overlying material of interest. “A single graphene layer helps to increase imaging sensitivity compared to other substrates,” she said. Stokes cautioned that contamination could be a problem, for which “carbon is notorious”.
As for studying atoms, Stokes believes the technique would be of limited use because it is “difficult to do and open to misinterpretation”.



