Physicists in the US have discovered that electrons flowing in carbon nanotube-based circuits dissipate energy in very different ways from electrons flowing through devices made from conventional semiconductors such as silicon. The findings reveal processes of heat conduction that were never previously thought important and could influence the types of materials chosen for the next generation of electronic devices in order to prevent them from overheating.
In conventional semiconductor devices, different layers of material are always joined by chemical bonds. This provides continuity for heat flowing through such devices, making them relatively easy to cool. Many researchers believe that future generations of electronic devices could be made from carbon nanotubes — tubes with walls just one atom thick — which could enable much smaller feature sizes and hence much better computing performance. However, nanotubes do not bond chemically to adjoining structures, which suggested that it should be very difficult to remove heat from such devices.
Bonding not needed
But now Phaedon Avouris and colleagues at the IBM Thomas J Watson Research Center in New York and researchers at Duke University in North Carolina have found that electrons in nanotubes can dissipate energy straight to an adjacent substrate even though it is not chemically bonded.
The team has also found that current-carrying electrons in nanotube devices do not undergo the normal process of “thermalization”, in which a material’s thermal vibrations reach statistical equilibrium (Nature Nanotechnology doi:10.1038/nnano.2009.22).
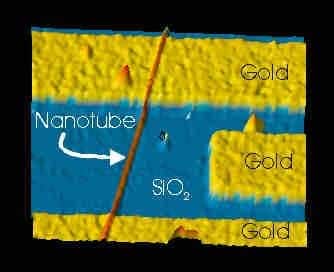
Avouris and team studied a carbon nanotube on a silicon-dioxide substrate, an arrangement that acts like the active channel of a field-effect transistor. They have used a variety of techniques including Raman scattering, in which the energy of scattered light reveals the different temperatures or “modes” of vibration of the nanotube lattice.
Normally when a current passes through a semiconductor the electrons bump into nearby atoms, which begin to vibrate in a certain mode. This mode then gradually transfers its energy to atoms at lower temperature modes until, at thermalization, all atoms are vibrating in statistical equilibrium.
The researchers have shown that, in nanotubes, thermalization does not take place; the atoms continue to vibrate in the same mode and statistical equilibrium is never reached.
Just as surprising, however, is that the lack of chemical bonding to the substrate does not inhibit heat conduction. The team has shown that when the electrons collide with atoms in the silicon dioxide, which is a polar material, the subsequent shift in position of the atoms generates an electric field that extends beyond the substrate and into the nanotube. When the nanotube’s electrons interact with this field they are able to dissipate energy straight to the substrate.
Overlooked effect
Scientists were aware of this process of remote heat conduction before, but had never considered it important because they had focused on 2D and 3D materials in which the effect is much weaker. But Avouris told physicsworld.com that the other unusual mechanism — the absence of thermalization — could exist in other materials, and that it may have been overlooked because researchers have not had the right observational tools.
“Understanding this dissipation mechanism in detail is important, especially if nanotubes are someday employed in electronic circuits,” says Adrian Bachtold, a nanoelectronics researcher at the Autonomous University of Barcelona. “Indeed, a better understanding is the first step to be able to engineer the dissipation pathway. For instance, it may be possible to find tricks to enhance the current in the ‘on’ state of the transistor, [which would be] good for rapid circuits.”
The US team is now investigating similar effects in graphene, a one-dimensional “chickenwire” lattice of carbon atoms rather like an unrolled nanotube. Avouris says his team knows that the same mechanisms occur in graphene, but expects some “curious effects” due the material’s larger footprint on the substrate.