Scientists in Germany have shown that a suspension of particles can be transformed from a viscous fluid to an elastic gel by adding a small quantity of a second liquid – as long as the second liquid does not mix with the bulk fluid. They say that the second liquid binds the particles more tightly together, and found that this enhanced binding takes place even when the liquid itself adheres poorly to the particles. Applications of this work, say the researchers, include lighter and cheaper foams as well as improved manufacturing of paints and other suspensions.
Being able to control the flow of suspensions – small, solid particles dispersed in a fluid – is important in the manufacture of many commercial products, such as coatings and foodstuffs. For example, it is better if paint is less viscous when it is being mixed during production, but more viscous when in its finished state so that it sticks to walls and does not drip.
In the latest research Erin Koos and Norbert Willenbacher of the Karlsruhe Institute of Technology have demonstrated a new and practical method for adjusting the viscosity of a suspension. In their experiment, they first dispersed hydrophilic (or water-attracting) glass beads, each about 25 µm in diameter, into an organic solvent. Then they added water to this suspension so that it made up just 1% of the suspension by weight. When they stirred, the initially viscous fluid transformed into a gel-like material.
Water builds bridges
This transformation has been known about for many years, and occurs because the water wets the particles – in other words, it tends to adhere to the surface of the hydrophilic particles more readily than does the organic liquid and so forms a thin film around them. When two particles get close enough, the water’s surface tension then dictates that it becomes energetically favourable for the coatings of water to join up and form a bridge, so binding the particles together and creating a network that makes the suspension more rigid.
What the researchers also found, however, was that the reverse can happen. When dispersing hydrophobic (water-repelling) glass beads into the solvent and then adding 1% water, they discovered that the initially viscous suspension becomes gel-like upon stirring. In other words, adding a small quantity of a substance that wets less well than the solvent, rather than better, also binds the beads together to form a more-or-less rigid network.
This process has also been observed before. For example, adding water to melted chocolate causes the latter to solidify even though the solvent in the chocolate – cocoa butter – wets the cocoa particles more readily. What Koos and Willenbacher have done is to demonstrate that this is a general phenomenon, having subsequently found that the process occurs in a wide variety of fluid and particle combinations. They were also able to pin down the mechanism responsible, and show that it is essentially the same process as occurs when introducing a superior wetter, but in reverse.
Rotating plates
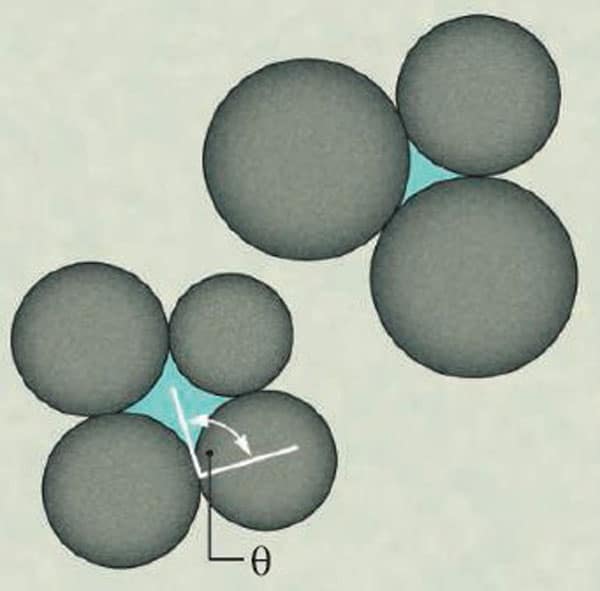
In their experiment, the researchers measured the amount of force needed to set two parallel metal plates rotating relative to one another when a suspension is placed between them. They found that the force required increased markedly as the second, non-mixable fluid was added to the suspension – in other words the “yield point” of the suspension increased – and they found that this increase is just what would be expected were the particle binding determined by surface-tension, or “capillary”, effects. In this case, however, the water does not coat the particles but instead seeks to minimize its total area of contact with the particles and the bulk fluid, which leaves the water enclosed by particles (see figure).
Koos says that this effect can also be seen when building sandcastles. Adding a little water makes sand firmer because it creates bridges between the sand particles. The inter-particle binding increases as more water is added but when the sand finally becomes saturated with water, and there is no longer any air present, the sand particles simply slosh around in the water. But adding back in a small amount of air – the inferior wetting fluid – provides nuclei around which the sand particles can agglomerate.
According to Koos, this ability to enhance rigidity by adding small quantities of either a superior or inferior wetter could improve the industrial manufacture of suspensions. For example, she says, they found that foam can be made by dispersing PVC particles in water and then adding a tiny amount of oil. This requires less PVC than the traditional approach of dispersing PVC directly into oil, meaning that the process is cheaper and the resulting foam is lighter, which could make it attractive in the manufacture of lightweight building materials and insulating foams.
Wilson Poon at the University of Edinburgh in the UK points out that while other groups are studying the effects of adding a small amount of a second, unmixable liquid to suspensions, this latest work provides a striking demonstration of the potential of such an approach. And he agrees that the results obtained by Koos and Willenbacher indicate that capillary forces play a dominant role. “Since it is very difficult to eliminate moisture entirely from oil-based systems, or oily impurities from aqueous systems, it is possible that the effects presented here are widespread, but not widely recognized as such,” he says. “It is always tempting to think that the odd 0.3% impurity doesn’t really matter. In fact, it may matter, and matter hugely, either for good or for ill.”
The work is described in Science 331 897.