Researchers in Singapore have found that carbon-60 molecules, or “buckyballs”, can be used to make graphene quantum dots that are geometrically well defined. Such structures could be ideal in next-generation electronics as single-electron transistors in nanoscale circuits.
Graphene is a 2D sheet of carbon just one-atom-thick, and is usually made by cleaving small crystals of graphite. At the molecular level it looks like a sheet of chicken wire – a spread of joined-up benzene rings.
The “wonder material”, as it is sometimes called, is often touted to replace silicon as the electronic material of choice thanks to its unusual electronic, thermal and mechanical properties. These include the fact that electrons in the material behave like relativistic particles that have no rest mass and can therefore travel at speeds of around 106 m/s. These properties remain even when graphene devices are scaled down to a few benzene rings.
Getting the most out of graphene
Until now, researchers have only been able to make transistors from ribbons of graphene but such long shapes do not maximize the conductivity of the material. Transistors made from structures that can confine electrons quantum mechanically could be much more effective. One such structure is quantum dots, which are nanoscale objects comprising several thousand atoms that form tiny compound semiconductor crystals. But existing “top-down” methods to fabricate dots from ribbons, for example, are still quite complicated.
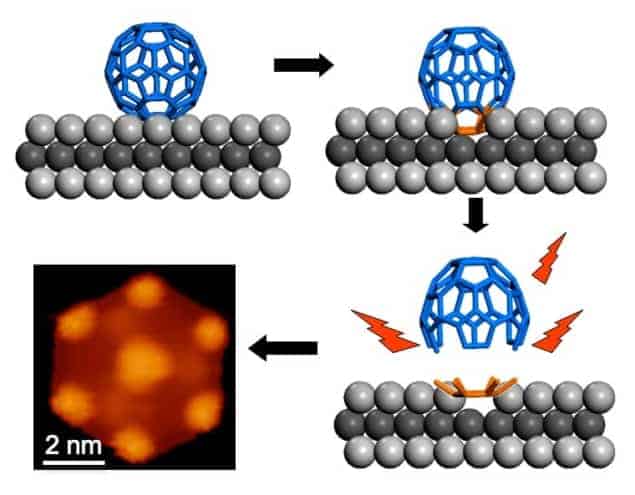
Now, Kian Ping Loh and colleagues at the University of Singapore have developed the first bottom-up approach to make graphene quantum dots smaller than 10 nm in size using fullerene molecules as precursors. What is more, the dots produced are all regularly sized and all the same shape – unlike those produced using top-down techniques.
Loh and co-workers generated their quantum dots by decomposing carbon-60 molecules at high temperatures on a ruthenium metal surface. The metal acts as a catalyst and causes the C60 to break down into carbon clusters. The researchers employed scanning tunnelling microscopy to observe how the carbon clusters diffused onto the metal surface and how they aggregated to form quantum dots.
Controlled manufacture
“By carefully controlling the density of the clusters on the surface, we were able to limit cluster aggregation and found that different-shaped clusters (flower-shaped and hexagonal) merged and crystallized into geometrically well-defined, hexagonal-shaped, graphene quantum dots at temperatures of around 825 K.”
To their delight, the researchers also found that the quantum dots had an energy gap that scales inversely with their size – that is, the smaller the dot, the larger its energy gap. This is an important result because graphene is normally metallic and engineering its band gap in this way to make the material semiconducting is one of the main goals of graphene research today.
Loh told physicsworld.com that he is confident that the technique might be used to produce large quantities of these dots in the future.
Spurred on by their results, the researchers now plan to isolate the quantum dots they made and fabricate devices from them. “Being chemists, we are also interested in studying the reactivity of the dots,” added Loh.
The work is detailed in Nature Nanotechnology.



