How many water molecules does it take to make the smallest possible ice crystal? Around 275: that is the conclusion of researchers in Germany and the Czech Republic, who have developed the first-ever technique for probing large clusters of water molecules. Their findings could help to shed light on the formation of ice high in the atmosphere.
Water clusters are assemblies of water molecules that are held together by intermolecular hydrogen bonds. Until now, most studies have focused on small clusters with 12 molecules or less and the structure of these objects bears little resemblance to bulk ice. In the past few years, researchers in Japan have developed a spectroscopy-based technique to probe water clusters containing up to 50 molecules. However, detailed structural analysis of clusters with 100–1000 molecules, where ice crystallization was thought to occur, was beyond the reach of these studies.
The main difficulty in analysing large water clusters is knowing exactly how many molecules they contain. This is done by mass spectrometry, which involves ionizing the clusters by hitting them with high-energy radiation, which can smash the delicate clusters into fragments. Furthermore, researchers would rather study neutral than charged water clusters because these are involved in most of the ice-crystallization processes in nature.
Doped water clusters
Now, researchers including Thomas Zeuch at the Institut für Physikalische Chemie in Göttingen, Germany, have found a way to analyse neutral water clusters containing hundreds of molecules. Their success lies in two clever tricks. First, each water cluster is doped with a single sodium atom. Using this highly reactive metal means that the doped water clusters are ionized more easily than pure clusters and ensures that the electron is liberated from the sodium atom rather than the neutral water cluster.
Second, before being ionized, the doped clusters are excited with infrared radiation. This increases their temperature, thereby altering their structure in such a way that further lowers their ionization potential. The clusters can then be ionized with a 390 nm ultraviolet laser, which has low-enough energy to avoid fragmentation. The sizes of these ionized water clusters are determined using time-of-flight (TOF) mass spectrometry.
Then, in order to probe their structure, the infrared spectra of the water clusters are calculated. Infrared radiation with wavenumbers between 2800 and 3800 cm–1 is used, corresponding to the vibrational (stretching) frequencies of oxygen–hydrogen bonds. This vibrational spectroscopy provides an insight into the arrangement of water molecules inside the cluster. For instance, it is known that crystalline ice has an absorption maximum at wavenumbers around 3200 cm–1, whereas amorphous ice and liquid water have maxima at approximately 3400 cm–1.
Turning water into ice
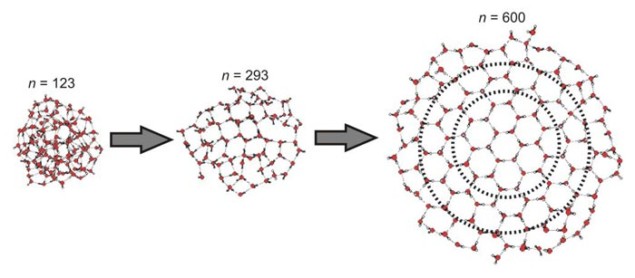
Zeuch and colleagues obtained infrared spectra for cluster sizes ranging from 85 to 475 molecules. As expected, there was a shift in the spectrum maxima towards lower wavenumbers as cluster size increased. The transition from 3400 to 3200 cm–1 began at around 275 molecules, with the first crystalline ice occurring in the centre of the cluster, forming a ring of six hydrogen-bonded water molecules in a tetrahedral configuration.
As the cluster size increased further, the crystalline core gradually grew. By 475 molecules, the infrared spectrum was dominated by the ice structure: the formation of the ice crystal was all but complete. This behaviour matched theoretical predictions made by a different group of researchers in 2004.
“It’s not such a surprise that water crystallizes when you bring together a certain number of water molecules,” says Zeuch. “But the question was ‘Where does this happen?’ We’ve now developed a technique that pinpoints the size range where crystallization takes place.”
Going stratospheric
This new technique could help scientists to understand cloud-formation processes in the Earth’s atmosphere. “There are regions in the stratosphere without any nucleation sites where ice crystals are formed directly from water molecules,” says Zeuch. “The dynamics of this process could now be modelled in more detail.”
“These are really exciting results,” says Francesco Paesani, a chemist at the University of California, San Diego, who studies water clusters. “Nanometre-sized water particles play an important role in the atmosphere and ice crystals can be found in many types of clouds. Therefore, understanding how water clusters crystallize provides fundamental insights into cloud formation and properties, which in turn influence the Earth’s radiation budget and climate.”
Zeuch also believes that the research will help scientists to better model the interactions between water clusters in molecular-dynamics simulations. Understanding exactly how these water clusters behave in bulk water is one of the key goals of these models and one of the great unsolved problems in chemistry.
The research is described in Science.