Tiny gold particles called nanocages that emit Cerenkov light could be used to image tumours and deliver drugs to destroy them at the same time. That is the claim of researchers in the US, who have detected Cerenkov light from within live mice that had been injected with the nanoparticles. The nanocages are among the very first reported “theranostic” nanoparticles that have the potential to fulfil both therapeutic and diagnostic roles in medicine.
Gold nanocages are tiny structures with hollow interiors and ultrathin porous walls. They are of particular interest to medical researchers because they do not interact with biological materials and can therefore be used within the body. Nanocages can also be designed to absorb and scatter light in the near-infrared (NIR) region of the electromagnetic spectrum. Light at these wavelengths (700–900 nm) can penetrate deeply into soft biological tissue and so is perfect for optical imaging based on photoacoustic and optical-coherence tomography.
Younan Xia at the Georgia Institute of Technology, together with Yongjian Liu from Washington University School of Medicine and Cathy Cutler at the University of Missouri, have done experiments that suggest that gold nanocages containing radioactive gold-198 could also be used as contrast agents in luminescence imaging, while in addition carrying drugs to tumours.
Cerenkov light from tumour cells
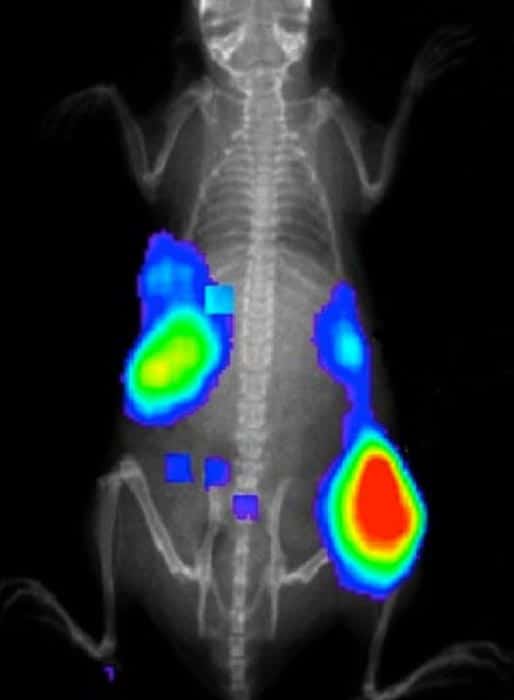
Gold-198 undergoes beta decay and emits a fast-moving electron, which in turn creates Cerenkov radiation in the form of visible and NIR light. When the nanocages are injected into the mice, they appear to accumulate in tumour cells, and therefore the Cerenkov light can be used to locate tumours. To demonstrate this, the researchers detected the Cerenkov light emitted by gold nanocages in live mice using a commercial imaging system.
Normally, such tumour markers are made to give off light by illuminating the region of interest with an excitation light source. This can sometimes cause healthy tissue to emit the same sort of light as a tumour – a problem that could be minimized by using Cerenkov-emitting nanocages.
No separation anxiety
Another important benefit of using a radioactive isotope of gold is that its presence does not alter the chemical properties of the nanocages and the isotope is unlikely to separate from the nanocage. “The radioactive gold-198 nanocages emit Cerenkov light but the physicochemical and other properties of these nanostructures remain unchanged,” explains team member Yucai Wang. “Our approach is thus a new and robust way to label a nanoparticle. In conventional radioisotope labelling (where the isotope is attached to a nanostructure by chemical means), there is always the concern that the label will ‘come off’ during treatment. By incorporating gold-198 atoms into the walls of the gold nanocages, such an issue does not exist anymore.”
The technique developed in this research could be used for diagnosing cancer and to guide nanoparticle delivery for treating tumours, Wang says. The latter could be achieved by adding targeting molecules, such as peptides, to the gold nanocages – something that the team is now investigating. The hope is that this could offer a new and improved way of delivering drugs to cancer cells that could be used in photothermal treatment, chemotherapy or a combination of the two.
The current work is reported in Nano Letters.



