Silicon nanoparticles could be used to produce hydrogen almost instantly, as they react with water, according to researchers at the University at Buffalo (SUNY) in New York. The reaction does not require any heat, light or electricity and the hydrogen generated could be used to power small fuel cells. The technology could come in handy as a “just add water” approach to produce hydrogen on demand, says the team. In essence, the technique recovers some of the energy that goes into refining the silicon and producing the nanoparticles in the first place.
Splitting water to produce hydrogen is a clean and renewable way to produce energy, and traditional techniques to split water include electrolysis, thermolysis and photocatalysis. Water can also react with bulk silicon to produce hydrogen, but this route has been little studied because it is slow. In theory, silicon can release two moles of hydrogen gas per mole of silicon (or 14% of its own mass in hydrogen). Silicon is also abundant on our planet, has a high energy density and does not release any carbon dioxide when it reacts with water.
Faster reaction rates
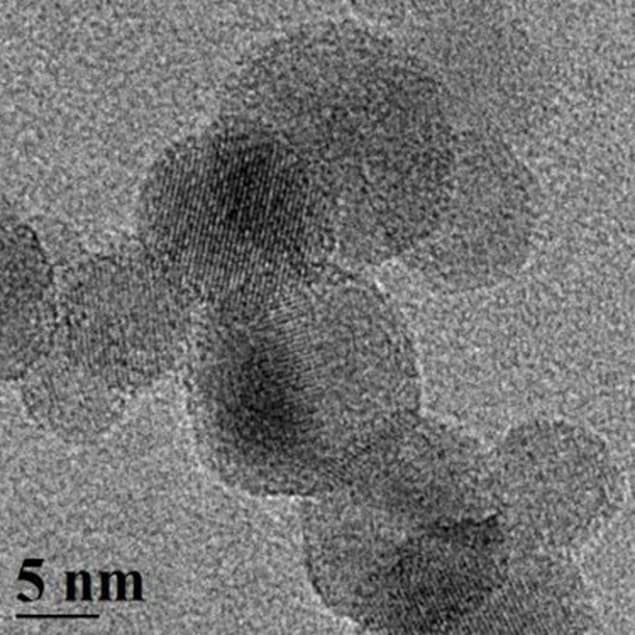
Thanks to their high surface to volume ratio, silicon nanoparticles should naturally generate hydrogen much more quickly than bulk silicon. Now, a team led by Paras Prasad and Mark Swihart at Buffalo has shown that the increase in reaction rate is much greater than would be expected based on increased surface area alone. In fact, nanoparticles 10 nm in diameter appear to produce hydrogen in under a minute, compared with around 45 minutes for nanoparticles that are 100 nm. The 10 nm particles are also 1000 times faster at producing hydrogen than is bulk silicon.
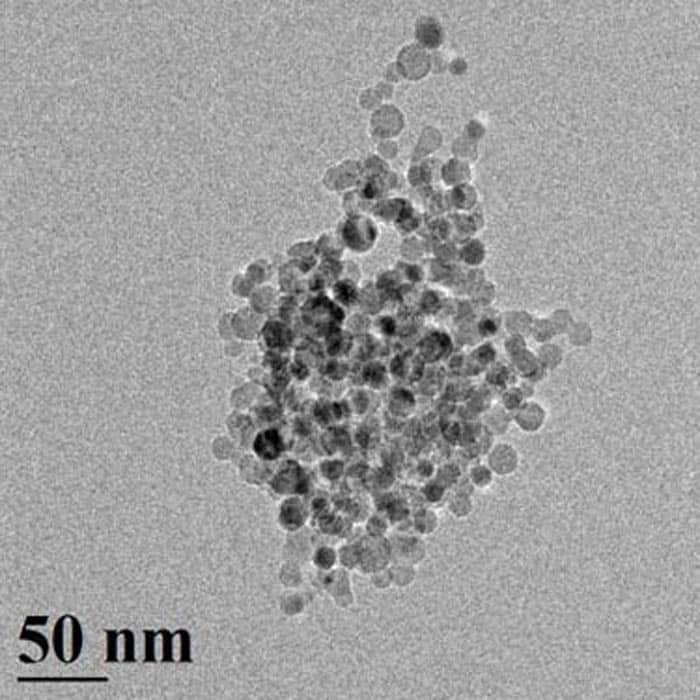
According to the SUNY team, the difference in hydrogen production rates between the 10 and 100 nm-sized silicon particles is much greater than can be accounted for by the difference in the surface areas of the particles. To understand this difference, the researchers conducted experiments in which they stopped the reaction before all of the silicon had been fully consumed. As the reaction proceeds, the 10 nm silicon particles reduce in size but do not change shape and remain roughly spherical.
The 100 nm particles, on the other hand, do not uniformly reduce in size but form hollow shells or capsules with walls consisting of a few monolayers of silicon. These walls then slow down the water–silicon reaction because they provide an extra layer through which the reactants must diffuse. Particles that are initially larger also have less surface area per unit volume.
Ideal for powering portable devices
“With further development, this technology could be ideal for powering small portable devices and might even replace bulky gasoline or diesel generators in the future,” says Prasad.
“A typical silicon generator could comprise a small hydrogen fuel cell and some plastic cartridges of silicon nanopowder, to which water would be added when needed, to produce energy,” adds team member Folarin Erogbogbo.
Although the technique could probably not be used to generate large amounts of hydrogen, the overall efficiency of the process could be quite competitive with primary batteries and other sources of portable power, which makes it interesting for these applications, Swihart told physicsworld.com.
The researchers have already successfully tested their technique in a small fuel cell that they used to power a fan. They are now planning to study the hollow nanostructures formed by the reaction of the larger silicon particles in more detail and look at how hydrogen can be produced when silicon nanoparticles are mixed with other materials, such as alkali hydrides. “These hollow ‘nanoballoons’ may have interesting applications in other areas such as anodes for lithium-ion batteries,” explains Swihart. “Alkali-metal hydrides react with water to release hydrogen and produce alkali-metal hydroxides (such as sodium hydroxide, for example) needed to catalyse the silicon reaction with water. On their own, the metal hydrides are air reactive and unstable, but coating them with silicon nanoparticles might let us increase the hydrogen generation capacity of the system while maintaining an air-stable, easy-to-handle material.”
The research is published in Nano Letters.