Physicists in California have combined infrared spectroscopy with scanning tunnelling microscopy to create a new technique that measures the subtle changes that can happen to molecules when they stick to a surface. The method promises to improve our understanding of how molecules behave on surfaces and could be used to help develop better surface catalysts for industrial processes. The team also believes that the technique could be further developed to map the locations of surface molecules on a nanometre scale.
Since its invention in 1981, the scanning tunnelling microscope (STM) has become an important research tool for physicists, chemists and materials scientists. It works by scanning an atomically sharp tip very near to the surface of interest and monitoring the electrical current flowing between the two. While a STM can spot individual atoms and molecules on a surface, it cannot directly distinguish between different chemical species – with two or more very different molecules appearing the same in an STM image.
Infrared spectroscopy, on the other hand, is very good at identifying molecules. It works by shining broadband infrared light onto a sample and each type of molecule that is present is identified by how it absorbs the light at a distinct set of frequencies. But because the technique relies on light with relatively long wavelengths – about 1 μm or longer – it cannot pinpoint the locations of molecules on the nanoscale.
Expand and crash
Several attempts have already been made to combine infrared spectroscopy with a STM. However, they have all had limited success because the infrared light heats the tip, causing it to expand and crash into the surface.
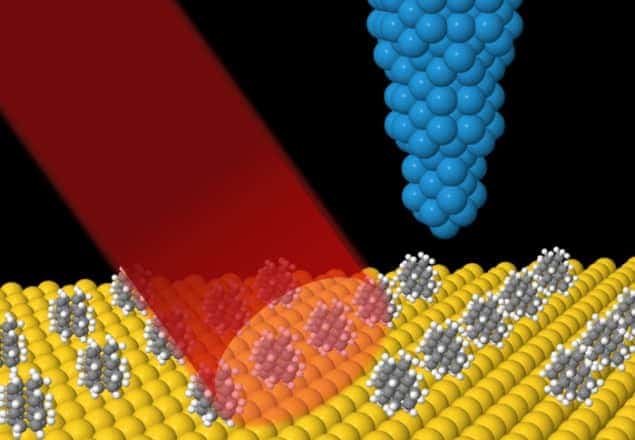
In this latest work, students in Michael Crommie‘s research group at the University of California, Berkeley (UCB) have taken a different approach to this problem. The team used a custom-built tunable infrared laser designed by Feng Wang, who is also at UCB, to irradiate a gold surface that is partially covered with single molecules of either [121]tetramantane or [123]tetramantane. It then positioned the tip of a STM above the gold surface about a millimetre away from the region that was illuminated by the laser. This is far enough away to avoid heating the tip.
The researchers found that when the laser’s frequency equalled one of the absorption frequencies of the adsorbed hydrocarbon, the tunnelling current between surface and tip increased. The team believes this is because when surface molecules absorb infrared light, the energy rapidly dissipates into the gold substrate as heat. This heat, the researchers suggest, causes the gold to expand slightly, thus bringing the surface closer to the needle and increasing the tunnelling current.
Very good resolution
By measuring the precise frequencies at which the tunnelling current increased, the researchers could identify the spectral fingerprints of either [121]tetramantane or [123]tetramantane, allowing them to identify which of the two molecules was adsorbed onto the gold substrate. Furthermore, they found that the spectral resolution of the technique is much better than previous STM-based methods.
By comparing the absorption spectra of molecules on the surface with those of the same molecules in a bulk sample, the team could deduce important information about how the surface-bound molecules interacted with each other and with the substrate.
There is a catch, however. Because the microscope tip has to be placed outside the laser spot, it detects an averaged signal from all the irradiated molecules. “We have not yet been able to perform infrared spectroscopy on a single molecule,” says team-leader Crommie, “but that’s something we want to do in the future.”
I’m really amazed that you can detect the energy from 1 mm away – that’s a distance of millions of atoms
Ludwig Bartels, University of California, Riverside
“I’m really amazed that you can detect the energy from 1 mm away – that’s a distance of millions of atoms,” says Ludwig Bartels, a STM expert at the University of California, Riverside who was not involved in the current research. He believes that the research marks “a paradigm shift in our understanding rather than an incremental increase”. However, he is sceptical of the researchers’ simple explanation that the signal is caused by the expansion of the substrate, saying he would be “truly amazed” if the amount of heat that was transferred to the material by the infrared photons caused detectable expansion 1 mm away. Instead, he suspects a more complex, as-yet-undocumented process might explain the energy transmission through the surface.
He also doubts the researchers’ prospects of achieving single-molecule vibrational spectroscopy using this method, suggesting that the signal from a single molecule would almost certainly be too weak to be detected without bringing the tip so close that it would be heated by the laser. He believes, however, that a proper understanding of how the energy radiates through the surface of the material could potentially have a major impact on the future of microelectronics.
The research is described in Physical Review Letters.


