A new mathematical model of cardiac muscle tissue could provide important insights into a heart condition called atrial fibrillation, which is a leading cause of strokes. The model was created by scientists in the UK, who hope that it could help to pinpoint troublesome regions of the heart so that doctors can destroy them.
During a regular heartbeat, blood travels from the upper chambers (the atria) into the lower chambers (the ventricles) and on around the body. Cardiac muscle pumps blood by contracting in response to electrical impulses, with each fibre-like muscle cell being excited to contract by a neighbouring muscle cell that itself has just been excited by a neighbour. In a healthy heart, these contractions move across the muscle as plane waves. In contrast, fibrillation involves the emergence of chaotic spiral waves that cause the heart muscle to quiver spasmodically rather than pumping blood effectively. Ventricular fibrillation cuts off the body’s blood supply and can very rapidly lead to cardiac arrest and death. Atrial fibrillation, the subject of this latest research, is less dramatic, but if blood is not efficiently expelled from the atria, it can begin to coagulate into blood clots. As a result, atrial fibrillation is the largest single cause of stroke.
A common treatment is to insert a catheter electrode into a particular part of the heart thought to be responsible for a patient’s atrial fibrillation and use radio waves to destroy it. However, identifying the regions responsible for initiating fibrillation remains a challenge, and outcomes are highly variable. Now, physicists Kim Christensen and Kishan Manani, together with cardiologist Nicholas Peters of Imperial College London, have constructed a simplified schematic model of how regions of poor transverse connectivity between cells affect the propagation of planar wavefronts in cardiac tissue.
Going backwards
Occasionally, a cell fails to contract in response to its neighbour, and this can create a wave that attempts to propagate in the direction opposite to the forward propagating wavefront. After a cell has been excited, it cannot be excited again for about 200 ms. If the cells are very well connected, then almost all of the cells will have been excited by the forward-propagating wavefront. This means that a rogue backward-moving wave will die out quickly because it cannot find a path through this temporarily unexcitable region. As the tissue becomes less well connected, however, more cells remain unexcited, and this provides more opportunities for backward waves to twist their way around the heart’s muscle.
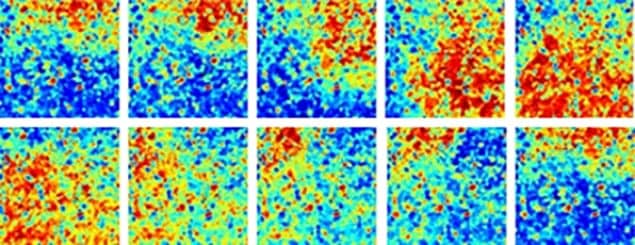
The Imperial team found that the system undergoes a sharp transition as the connectivity is varied in the model. When more than a certain fraction of the cardiac cells are transversely connected to their neighbours, the researchers’ simulation shows only plane waves in the muscle. When the fraction of connected cells was just below this threshold, they observed isolated spiral waves that spontaneously formed but did not propagate through the tissue. When the fraction was further below threshold, however, they found many more such waves spontaneously forming and, crucially, stimulating other spiral waves that permeated throughout the tissue – the same behaviour as seen during persistent atrial fibrillation.
The work suggests that in a heart with a connectivity fraction near the threshold value, the sources of the spiral waves could be detected and destroyed – and normal cardiac rhythm would resume. “If patients have been in atrial fibrillation for many years, it’s anticipated that they’d have a lot more fibrosis,” says Manani. Treatment of these patients is less successful.
Complex physiology
However, biophysicist Flavio Fenton of Georgia Institute of Technology says that the work reveals little that had not been demonstrated in a 2002–2005 series of papers by Gil Bub, then at the State University of New York Downstate Medical Center, and colleagues in Canada. “The atrium is not smooth: it is a very complex physiological structure,” he explains. “There are networks of thin muscles branching and crossing, irregular boundaries, fibre anisotropy, all of which affect significantly conduction.” This paper, he says, considers the electrical connections between the cells with no consideration of the electrophysiology of the cells themselves – something that Bub and colleagues have already done.
Bub himself, however, is more positive. “When I was writing those papers, if someone had told me that you could take a real piece of tissue that was showing a spiral wave, ablate that spiral core, and then end up with no activity, I would have said that’s completely nonsensical,” he says. However, results from a recent clinical trial have shown exactly this. “Everybody’s really surprised,” he adds. “This paper is interesting because the simulations back up this idea that you can have these localized sources, and they show a very reasonable way to explain the kinds of behaviours that the surgeons are seeing.”
The research is published in Physical Review Letters.