While proton therapy is a very promising way of treating cancer, researchers are keen to ensure that the beams have indeed hit their target. While this could be achieved using positron emission tomography (PET scanning), it needs to be done immediately after the therapy has been carried out, before the radioactivity within the patient decays and becomes distorted by biological “washout”. Now, researchers in the US have developed a more accurate extrapolation of radioisotope distributions at the time of irradiation using a kinetic model applied to dynamic PET scans done soon after irradiation. According to the researchers, their approach provides a patient-specific alternative to generic “washout factors” derived from animal studies, which have limited accuracy.
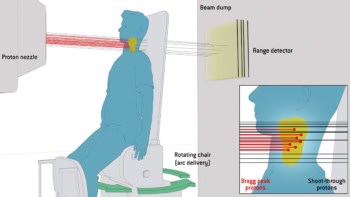
Proton therapy makes use of extremely precise proton beams that are directed at tumours to destroy them while avoiding any surrounding healthy tissue. PET shows promise as a means to verify proton-therapy delivery, by imaging positron emitters generated by a proton beam. However, in vivo radioisotope distributions are distorted by biological washout, which varies between patients and increases with the delay between treatment and scanning, thus presenting a major challenge.
A kinetic model
The model – developed by Kira Grogg of Massachusetts General Hospital (MGH), together with colleagues from MGH, Harvard Medical School and Yonsei University in Wonju, South Korea – describes the variation in concentration of activity for a given molecule containing a positron emitter, taking into account both the isotopes that are generated during the therapy and their subsequent decay or clearance. This clearance is determined by both physical decay and biological washout that varies according to the radioisotope’s molecular form.
“Our method can be used to improve PET monitoring of proton therapy by removing biological factors that affect the comparison of delivered to expected distributions,” says Grogg. “This is a step in making the process more accurate and reliable, and should eliminate the need for empirical washout factors when imaging is done soon after treatment.”
Several radioisotopes are generated during irradiation – including nitrogen-13 (13N) and carbon-11 (11C) – in a variety of molecular forms. However, 80% of the PET signal originates from oxygen-15 (15O), and water dominates the body’s composition. Consequently, the researchers’ model assumes a dominant contribution from the 15O present in water molecules with a correction for other radiolabelled molecules. By fitting the model with dynamic PET data, 15O production and clearance can be carefully and accurately determined.
Phantom and animal validations
Grogg and colleagues first tested their model for the simple case of a “phantom” (a specially designed object used in medical imaging to study, evaluate and tune the performance of imaging techniques), without the presence of biological washout. The phantom, made up of three materials with known elemental compositions, was irradiated with a passively scattered proton field and promptly imaged with a mobile PET scanner for 30 minutes with 1 minute frames. The team found that the fitted model correctly predicted physical decay constants for 15O, 13N and 11C.
Biological washout was introduced by scanning the thigh of a live rabbit. The scan was then repeated after the animal was sacrificed, leaving only physical decay. Using the model, the two scenarios gave 15O production rates within 2% of one another and the clearance rate was also consistent, further validating the model.
“Currently, we are collecting patient images with our mobile PET/CT, which we can bring into the treatment room,” says Grogg of ongoing research at MGH. “We hope to demonstrate the feasibility of our method in human studies, and, in particular, show an improvement in the comparison between measured and simulated PET images, as an initial step to clinical implementation.”
For full clinical implementation, a dedicated in-room scanner that reduces the time between treatment and imaging is needed, ideally using a partial-ring detector to acquire data during treatment, or a full-ring detector that can be moved into place immediately following irradiation, says Grogg.
The research is published in the International Journal of Radiation Oncology Biology Physics.