Electron pairing without superconductivity has been seen for the first time by a team of physicists in the US. Confirming a prediction made in 1969, the electron pairs were spotted in strontium titanate using a single-electron transistor. The observation could provide useful insights into the nature of superconductivity, and perhaps even help in the design of new high-temperature superconductors.
In a conventional superconductor, electrons with opposite spin come together to form Cooper pairs that pass through the atomic lattice without scattering. This interaction occurs because the presence of one electron pulls in positive ions from the lattice, and this in turn attracts the next electron. These pairs then interact with each other to form a condensate from which individual electrons cannot be easily scattered. For this to work, however, the electrons have to be relatively close together. This is not the case in strontium titanate, which has a very low electron density yet is a superconductor at temperatures below a critical temperature (TC) of about 300 mK.
Unexplained mechanism
In 1969 David Eagles of the NASA Electronics Research Center in Massachusetts calculated the properties of a hypothetical material that had an unspecified physical mechanism that caused electrons to pair up but not form a condensate. He found that if such a material was cooled below a critical temperature, these pairs could form a condensate and the material would become a superconductor. This had remained unobserved ever since, but the mechanism has been suggested as a possible explanation for certain unexplained properties of high-temperature cuprate superconductors.
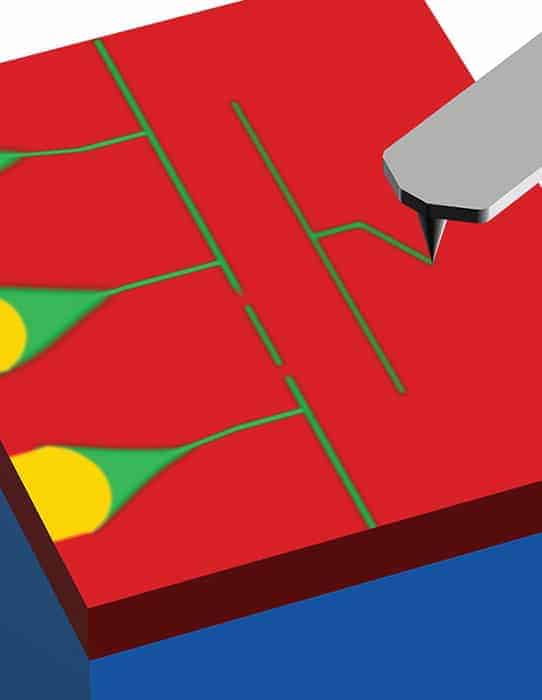
Now, Jeremy Levy of the University of Pittsburgh and Pittsburgh Quantum Institute, both in Pennsylvania, and colleagues have seen the first evidence for electron pairing at temperatures well above TC. To do this, the team fabricated single-electron transistors at the interface between strontium titanate and a layer of lanthanum aluminate. This was done using an atomic force microscope to create nanometre-scale source, drain and gate electrodes separated by a quantum dot.
By altering the voltage on the gate, the researchers could add and remove electrons one by one, to and from the quantum dot. They measured how the conductance between the source and drain varied with the voltage applied to the gate. When the electron density in the dot was high enough and the electric field was very low, the system effectively behaved as a superconductor. However, when the density was lowered, the team observed a series of repeated peaks and dips in conductivity as electrons were added and removed, as is expected with a single-electron transistor.
Crucial evidence
The crucial evidence came when the researchers applied a magnetic field. At fields above about 3 T, each peak in the conductance of the transistor split into two closely spaced peaks. This showed that electrons were not entering and leaving the transistor one by one, but two by two. This is proof that, even when the system was not superconducting, the electrons were still paired.
The researchers now want to investigate the physical mechanism that makes the electrons pair up. Gaining a good understanding of this relatively simply system could further our comprehension of superconductivity in some more complicated materials. Levy believes that this knowledge could help physicists in their search for materials that are superconductors all the way up to room temperature – which is about 160° warmer that the highest known TC today.
More clear-cut
David Eagles is pleased to see the research. He points out that a group he was part of at CSIRO Division of Applied Physics in Australia found evidence for similar pairing in bulk strontium titanate doped with zirconium in the 1980s, although he says that “this case may be more clear-cut than mine”.
Christopher Bell of the University of Bristol commends Levy and colleagues on their experimental prowess: “This system is extremely complicated. Jeremy’s lithography technique is tough and they’ve had to work very hard to get this to work.” Kamran Behnia of EPSCI in Paris is also impressed by the results, but adds a note of caution about their interpretation. “This experiment shows electrons moving in pairs,” he explains. “Are these Cooper pairs? I think this has yet to be proved.”
The research is published in Nature.