A new mechanism that keeps a protein complex mechanically stable when stretched has been discovered by researchers in Germany, the US and Israel. The mechanism channels forces along paths perpendicular to the “pulling” axis in the structure and its discovery could lead to a better understand of how proteins and other large biological molecules resist external forces. The work may ultimately see the development of artificial mechano-active systems that might be used as scaffolds for tissue engineering or as components in engineered nanomaterials or protein-inspired machines.
Mechanical forces are fundamentally important in biological systems. Cells sense and respond to mechanical cues in their environment and react, for example, by modulating gene-expression patterns. Forces also play an important role in how cells join together to create larger structures such as tissues and organs. At the molecular level, such behaviour is governed by mechanically active proteins, which can sense and react to external forces by changing their shape and modulating their function in a number of ways.
Extremely stable
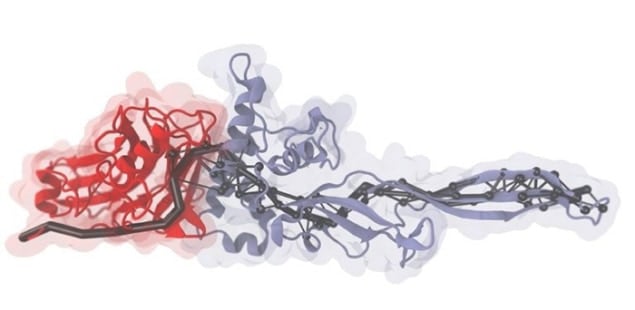
The researchers, co-led by Hermann Gaub of Ludwig-Maximilians University in Munich, used experimental and numerical methods to study mechanical properties of a multi-domain cellulosome protein complex. This complex is of great interest to researchers because it is known to be extremely stable when subject to mechanical forces.
Constantin Schoeler and colleagues in Munich used an atomic force microscope (AFM) to pull on a protein complex while monitoring how forces travel through the structure. Meanwhile, Klaus Schulten and Rafael Bernardi of the University of Illinois performed steered molecular dynamics (SMD) simulations using state-of-the-art supercomputers to calculate how forces propagate through cellulosome. After comparing their findings, the teams were able to identify the most probable paths that applied forces take through the molecules.
“Our results show that this mechanically stable complex uses an architecture that exploits simple geometrical and physical concepts from Newtonian mechanics to resist external forces,” say the researchers. “The analytical framework we describe provides a basis for developing a deeper understanding of how various mechano-active proteins function.”
Non-parallel routes
“As far as we know, this is a new concept in the biophysics field,” explains co-team-leader Michael Nash of the Center for Nanoscience at Ludwig-Maximilians University in Munich. “Our results imply specific force-propagation routes non-parallel to the pulling axis make the protein complex more mechanically robust.”
“Based on this work and other work from our group, we have developed a ‘toolbox’ of molecular modules based on cellulosomes that we could now use in a variety of biophysics experiments,” adds Nash. “We are now further investigating the fundamental properties of these remarkable molecules and looking into how we can exploit cellulosome proteins in diverse fields, from biomedicine to bioenergy.”
A better understanding of how proteins respond to external forces could provide important information to scientists who are trying to develop scaffolds for tissue engineering. These are artificial structures that provide a template for living cells to create tissues or organs. Such scaffolds must have very specific mechanical properties to create the desired tissue or organ.
The research is described in Nano Letters.
- This article first appeared on nanotechweb.org