A genetically engineered tissue that can be electrically excited by light has been developed by researchers in the US. The team believes its new material could be used as a simple model of the heart, allowing information about electrical malfunctions such as arrhythmias to be gathered. It could also lead to the development of biological computers that could be interfaced with the natural world.
The human heart is an extremely complex tissue, and when things go wrong with it, doctors and scientists often struggle to determine the causes of specific symptoms and devise appropriate treatments. Researchers are therefore trying to create simplified model systems for the heart to gain a better understanding of how it functions. A simplified model of the heart can be produced using electrically coupled cells, so that a change in the electrical potential of one induces a change in the potential of its neighbours. This allows electrical waves to propagate through the tissue – just as they do in a beating heart.
In this latest research, biophysicist Adam Cohen and colleagues at Harvard University based their model on human embryonic kidney cells. Such cells are not normally electrically excitable, and Cohen says they formed a “blank slate” for the study. The researchers edited the cells’ genomes so that the cells developed four ion channels – pores that can open or close to allow specific ions in or out of the cell, altering its electrical potential. First, they added a potassium-ion channel present in the heart to allow positive potassium ions (K+) out of the cell and lower the resting electric potential. Second, they added a sodium-ion channel – also present in the real heart – that opens when the potential increases. This allows sodium ions (Na+) into the cell, creating a runaway increase in voltage.
Red and blue light
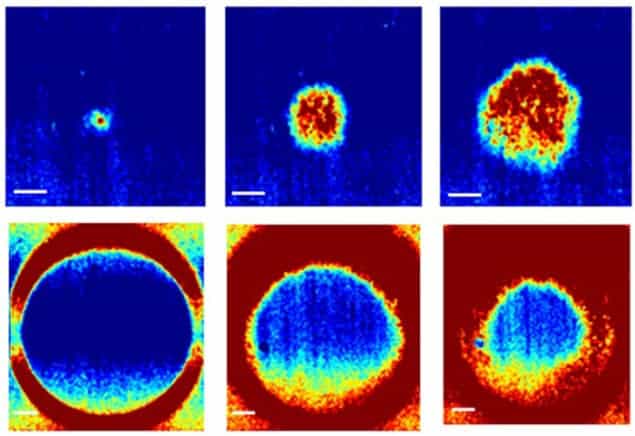
In equilibrium, the K+ channel holds the potential down, keeping the Na+ channel closed, and the cells stay negatively charged. However, an increase in applied voltage will open the Na+ channel, triggering a voltage spike that opens the Na+ channels of neighbouring cells and sends an electrical pulse like a heartbeat through the tissue. Finally, they added two light-responsive-ion channels that they had developed previously. One of these channels raises a cell’s potential in response to blue light, while the other creates a voltage-dependent fluorescence in response to red light. Together, these allowed the team to trigger and image the electrical waves in the tissue culture.
The researchers then measured various properties of their tissue culture, which revealed a few surprises. “If you drive a real heart too fast, it can go into an arrhythmia, where it starts to beat erratically,” explains Cohen. “No matter how fast we paced them, we were never able to trigger arrhythmias in these cells. There are elaborate feedback mechanisms that allow the heart to adapt to changes in demand, and it’s actually those feedbacks gone awry that lead to arrhythmias.” The researchers are now planning to add in other ion channels “one at a time”, to learn about their individual contributions.
Circulating waves
In addition to modelling the heart, the researchers showed that electrical circuits could be created and reconfigured dynamically in the tissue. After firing once, the Na+ channels are not re-armed until after the light is turned off, so permanently illuminating a region with blue light makes it unexcitable. Rings of excitable tissue could sustain continuously circulating waves in either direction for more than 1000 cycles. The waves could easily be stopped with blue light, however, before being launched in the opposite direction – thereby forming a rudimentary cellular memory.
In the future, more complex logical operations could be implemented using the tissue cultures, and these could be used to perform complex bioelectrical computations. However, the clock speed of such cellular computers would be limited to about 100 Hz by the recovery time of the cells – which is millions of times slower than standard computers. The researchers also point out that the tissues could be used in environmental sensors that detect the presence of specific chemicals. “One could put ion channels into these cells that are activated by different things one might want to sense in the environment,” says Cohen.
Gil Bub of the University of Oxford in the UK describes the work as “important”. “It’s a very nice reductionist approach with a tremendous amount of power for figuring out what specific [ion] currents are responsible for what types of behaviour,” he says. “The potential downside is that you don’t necessarily know what currents and what feedback loops are there in the intact system.” He says these kinds of experiments will be most useful if performed in tandem with experiments on the real heart.
The research is published in Physical Review X.