A new microscope lens that offers the unique combination of a large field of view with high resolution has been created by researchers in the UK. The new “mesolens” for confocal microscopes can create 3D images of much larger biological samples than was previously possible – while providing detail at the sub-cellular level. According to the researchers, the ability to view whole specimens in a single image could assist in the study of many biological processes and ensure that important details are not overlooked.
Laser-scanning confocal microscopes are an important tool in modern biological sciences. They emerged in the 1980s as an improvement on fluorescence microscopes, which view specimens that have been dyed with a substance that emits light when illuminated. Standard fluorescence microscopes are not ideal because they pick up fluorescence from behind the focal point, creating images with blurry backgrounds. To eliminate the out-of-focus background, confocal microscopes use a small spot of illuminating laser light and a tiny aperture so that only light close to the focal plane is collected. The laser is scanned across the specimen and many images are taken to create the full picture. Due to the small depth of focus, confocal microscopes are also able to focus a few micrometres through samples to build up a 3D image.
In microscopy there is a trade-off between resolution and the size of the specimen that can be imaged, or field-of-view – you either have a large field-of-view and low resolution or a small field-of-view and high resolution. Current confocal microscopes struggle to image large specimens, because low magnification produces poor resolution.
Stitched together
“Normally, when a large object is imaged with a low-magnification lens, rays of light are collected from only a small range of angles (i.e. the lens has a low numerical aperture),” explains Gail McConnell from the Centre for Biophotonics at the University of Strathclyde, in Glasgow. “This reduces the resolution of the image and has an even more serious effect in increasing the depth of focus, so all the cells in a tissue specimen are superimposed and you cannot see them individually.” Large objects can be imaged by stitching smaller images together. But variations in illumination and focus affect the quality of the final image.
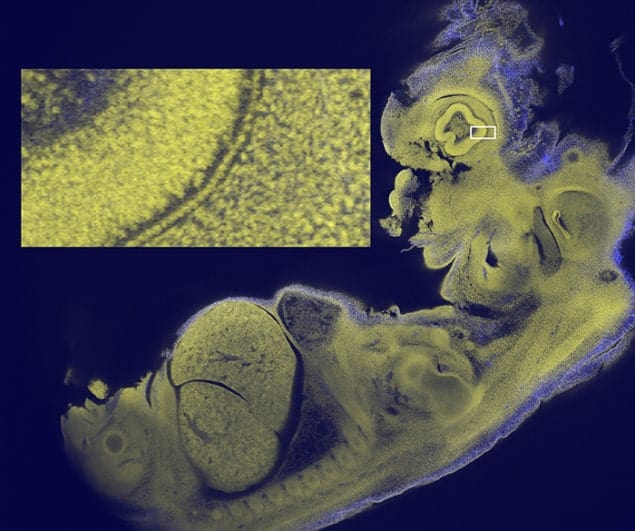
McConnell and colleagues set out to design a lens that could image larger samples, while retaining the detail produced by confocal microscopy. They focused on creating a lens that could be used to image an entire 12.5 day-old mouse embryo – a specimen that is typically about 5 mm across. This was to “facilitate the recognition of developmental abnormalities” in such embryos, which “are routinely used to screen human genes that are suspected of involvement in disease”, says McConnell.
Dubbed a mesolens, their optical system is more than half a metre long and contains 15 optical elements. This is unlike most confocal lenses, which are only a few centimetres in length. The mesolens has a magnification of 4× and a numerical aperture of 0.47, which is a significant improvement over the 0.1–0.2 apertures currently available. The system is also able to obtain 3D images of objects 6 mm wide and long, and 3 mm thick.
The high numerical aperture also provides a very good depth resolution. “This makes it possible to focus through tissue and see a completely different set of sub-cellular structures in focus every 1/500th of a millimetre through a depth of 3 mm,” explains McConnell. The distortion of the images is less than 0.7% at the periphery of the field and the lens works across the full visible spectrum of light, enabling imaging with multiple fluorescent labels.
Engineering and design
The lens was made possible through a combination of skilled engineering and optical design, and the use of components with very small aberrations. “Making the new lens is very expensive and difficult: to achieve the required very low field curvature across the full 6 mm field of view and because we need chromatic correction through the entire visible spectrum, the lens fabrication and mounting must be unusually accurate and the glass must be selected very carefully and tested before use,” explains McConnell.
The researchers used the lens in a customized confocal microscope to image 12.5 day-old mouse embryos. They were able to image single cells, heart muscle fibres and sub-cellular details, not just near the surface of the sample but throughout the depth of the embryo. Writing in the journal eLife, the researchers claim “no existing microscope can show all of these features simultaneously in an intact mouse embryo in a single image.”
The researchers also write that their mesolens “represents the most radical change in microscope objective design for over a century” and “has the potential to transform optical microscopy through the acquisition of sub-cellular resolution 3D data sets from large tissue specimens”.
Rafael Yuste, a neuroscientist at Columbia University in New York, saw an earlier prototype of the mesolens microscope. He told physicsworld.com that McConnell and colleagues “have completely redesigned the objective lens to achieve an impressive performance”. He adds that it could enable “wide-field imaging of neuronal circuits and tissues while preserving single-cell resolution”, which could help produce a dynamic picture of how cells and neural circuits in the brain interact.
Video images taken by the mesolens can be viewed in the eLife paper describing the microscope.