The optical properties of single-walled carbon nanotubes (SWCNTs) change when the tiny structures are filled with water. That is the conclusion of scientists in Belgium and the US, who attribute the change to a “quasi-phase transition” that occurs in the water – although the exact nature of the transition is unknown. The research points to a new technique for studying confined water molecules – which is crucial to various branches of science, but it is surprisingly difficult to do. The study could lead to better ways of delivering drugs in the body and even boost our understanding of quantum mechanics.
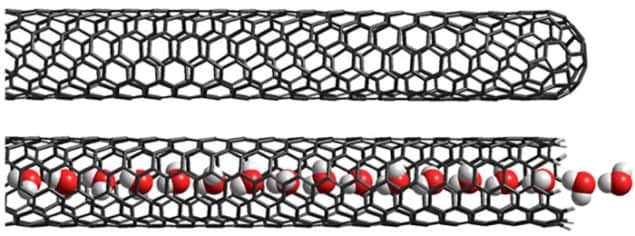
SWCNTs are hollow hair-like structures with walls that are one atom thick. Normally they are closed at both ends, but sometimes the ends can be open and Sofie Cambré of the University of Antwerp in Belgium and colleagues have previously shown that open SWCNTs rapidly fill with other molecules and hold on to them. Why this occurs is not well understood, but Cambré says “it seems the energy of a molecule inside a SWCNTs is much lower than when you have them separated.”
SWCNTs are naturally fluorescent, and the colour of the fluorescent light shifts if the nanotube is filled. “You need dedicated equipment to really see these very small shifts”, Cambré says. However, by measuring the shifts, researchers can potentially gain useful insights into the behaviour of confined molecules.
Single file
Cambré and her Antwerp team – together with Xuedan Ma and colleagues at the Center for Integrated Nanotechnologies, part of Los Alamos National Laboratory in New Mexico – looked at SWCNTs around 0.75 nm in diameter. This is large enough to accommodate water molecules in single file. They prepared an aqueous suspension of SWCNTs, causing the open SWCNTs to become filled with water. Then they used ultracentrifugation to separate empty and full SWCNTs. Both were dried to create films, and the filled SWCNTs retained their water during the drying process. The researchers then measured the change in each sample’s fluorescence spectrum as a function of temperature.
The team measured a gradual increase in the emission frequencies of both samples with temperature. This has been seen before and is believed to arise from strain resulting from thermal expansion. They also noticed a fairly sharp increase in the emission frequency of the filled SWCNTs at around 150 K, which was not present in the spectrum of the empty SWCNTs. The researchers attribute the increase to a “quasi-phase transition” in the chain of water molecules. Quasi because a true phase transition is not, strictly speaking, possible in 1D, explains Cambré. “If you just have a single row of water molecules, you cannot describe what is going on as freezing or boiling or anything like that.”
The exact nature of the transition is more puzzling. The researchers believe it is most likely a shift from a state in which the molecules form an orderly arrangement to one in which their orientation is random. However, it could also be a shift between two different ordered arrangements.
Water desalination
The observation of a type of phase transition in a 1D system has fundamental physics interest, Cambré says, but the research could also have applications in numerous other areas of science. The team is now studying the behaviour of water in other nanotubes with different diameters as well as the behaviour of other encapsulated fluids. Understanding confined fluids is a step towards controlling them, which could be useful in filtration applications. “If you could create a membrane that consisted only of nanotubes with one specific diameter, you would have a very selective means of transport,” says Cambré: “If, for example, only water can pass through and ions cannot, then you have desalination of water.”
The ability to transport molecules inside nanotubes and control their release could be used to deliverer drugs to where they are needed in the body while avoiding side effects elsewhere. The confinement of magnetic particles could even be useful for investigating magnetism on the quantum scale. “There are many interesting things we can do and we’re definitely excited about this,” says Ma, now at Argonne National Laboratory in Illinois.
Beautiful experiments
Jeffrey Fagan of the National Institute for Standards and Technology in Boulder, Colorado is impressed. “The important part of this work is that it demonstrates, with a very well defined experimental system, that the behaviour of the filler (in this case water) inside the nanotubes will likely be more complex than simply representing the bulk properties of that material,” he says. “The beauty of their experimental design is such that the effects of this complexity can be observed clearly. Contributions from other extrinsic effects on the nanotube spectra such as the external dielectric environment or strain can be eliminated through comparison of the water-filled nanotube population to the otherwise identical empty nanotube population.”
The research is published in Physical Review Letters.