The first observation of the low-temperature transformation of solid hydrogen into a metal – first predicted over 80 years ago – has been claimed by researchers in the US. The material needs further investigation – it is not clear whether it is a solid or a liquid – but some theoreticians have predicted exotic, and potentially useful, properties for metallic hydrogen such as room-temperature superconductivity. At least one leading high-pressure physicist, however, remains unconvinced by the results.
Hydrogen is a colourless diatomic gas under standard conditions. However, in 1935, Eugene Wigner and Hillard Huntington predicted that, at a pressure of 25 GPa (250,000 times atmospheric pressure) or higher, it would form an atomic, solid metal. This pressure was later shown to be hugely underestimated, as hydrogen becomes less compressible as its density increases. Liquid metallic hydrogen comprises the majority of the planets Jupiter and Saturn and this liquid metal can be produced by heating hydrogen up at high pressure until it crosses the so-called plasma phase transition. It was first observed in static experiments by Isaac Silvera and colleagues at Harvard University in 2016.
However, the so-called Wigner-Huntington transition, in which solid metallic hydrogen forms without heating at even higher pressures, had not been definitively observed, despite several suggestions that the material might have interesting properties. In 1968, Neil Ashcroft of Cornell University in Ithaca, New York, suggested that it could be a high-temperature superconductor. Then in 2011, David Ceperley and Jeffrey McMahon of the University of Illinois predicted that, at 500 GPa, the transition temperature would be well above room temperature.
Fade to black
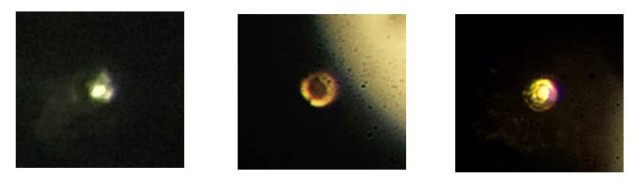
In 2016, Silvera’s team reported compressing hydrogen in a diamond anvil cell to 420 GPa – the highest static pressures then reported, in a paper on the arXiv preprint server. At 335 GPa, the sample turned from a transparent phase to a black one, but concluded that it was not metallic. Intriguingly, Mikhail Eremets and colleagues at Max Planck Institute for Chemistry in Mainz, Germany, published another arXiv paper in 2016 identifying a “possible metallic” phase in hydrogen at 360 GPa. Silvera and colleagues believe this is likely to be the same phase that they observed.
In the new research, Silvera and his colleague Ranga Dias modified their apparatus to increase the pressure even further. They found that at 495 GPa, the sample changed from black to highly reflective – which Silvera and Dias say is evidence that the hydrogen has become a metal. Numerous questions remain, however, such as the sample’s state: “It’s possible that at low temperatures, the ground state of hydrogen is a liquid,” says Silvera, “If it’s a liquid, then it’s all part of the same phase of liquid metallic hydrogen. If it’s a solid, which I think it is, then that’s interesting too.”
Silvera and Dias have maintained the sample stably at liquid nitrogen temperatures for about three months. They now intend to conduct a series of ever-more challenging tests such as Raman and X-ray scattering to determine its state and structure and resistance measurements to determine its electrical conductivity. Perhaps most tantalizingly, it wants to release the pressure to see whether it remains metallic: “It’s been predicted that metallic hydrogen is metastable,” explains Silvera. If it turns out to be a superconductor, this would be especially interesting, although what would happen to the transition temperature remains uncertain: “I would expect that, if it was a superconductor at very high pressure, and you released the pressure and it was metastable, the critical temperature would change somewhat, but probably not a great deal,” Silvera says.
There have been many false claims in the past, so I think everyone will look for confirmation and for more data about the new phase
David Ceperley, University of Illinois
Ceperley is cautiously enthusiastic: “The search for metallic hydrogen has been kind of a contentious field,” he says. “There have been many false claims in the past, so I think everyone will look for confirmation and for more data about the new phase.”
Eremets, however, is not convinced, saying “We observed much stronger evidence of metallicity but we did not claim that it was really metallic, just possibly metallic.” He criticizes the absence of repeated experiments and describes the techniques used to measure pressure as “ambiguous”, saying the true pressure could be anywhere between about 400-630 GPa. Finally, he criticizes the researchers’ reliance on reflectivity measurements as proof of metallicity without data on conductivity: “What they observe could be from a semiconductor,” he says, “Because narrow-gap semiconductors reflect very well.”
Silvera disputes this interpretation, saying that the reflectivity of a semiconductor should increase with temperature, whereas their material became more reflective as they cooled the material: “This is the expected behaviour for a metal,” he concludes.
The research is described in Science.