Physicists at the University of Kaiserslautern in Germany have observed how individual atoms diffuse through a gas for the first time, and how individual collisions between particles affect diffusion. The new study could help model diffusion in rarefied environments, such as thin layers of air in the upper atmosphere, in interstellar space or in vacuum systems.
Diffusion is the process whereby tiny particles uniformly disperse throughout a gas or liquid. Although these media are made up of individual particles, researchers usually describe diffusion as a continuous process.
Diffusion was first described by the Scottish botanist Robert Brown, who observed that grains of pollen appear to quiver as they zigzag through a liquid. This movement came to be known as Brownian motion and it allows substances to disperse and mix. Albert Einstein, in his seminal 1905 paper, explained diffusion at the microscopic level and showed that Brownian motion comes about thanks to collisions of particles with molecules of the surrounding medium.
Billions of collisions
In early studies of Brownian motion, the particles considered were much larger than the molecules in the medium. This means that billions of collisions are required to change the path of such particles. Not every collision is tracked in such studies. Rather, the collective effects of impacts are modelled as a randomly fluctuating process. When combined with the medium’s viscosity, the particle’s energy loss to its surroundings can be calculated. This approach is described by the Langevin equation, which can be used to calculate, for example, how a particle’s average speed changes over time.
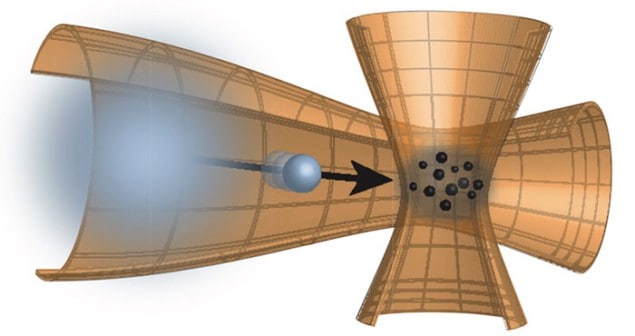
However, individual collisions are much more important when the particles have roughly the same mass as the atoms of the gas or liquid medium. To study this scenario, a team led by Artur Widera looked at how a few caesium atoms diffuse through a thin cloud of ultracold rubidium atoms held within an optical trap. Operating at a very low temperature drastically reduces collisions between the caesium and rubidium atoms so that their individual motions can be observed.
The experiment involves firing caesium atoms one by one into the gas. After a certain time delay the team froze the motion of the caesium atoms by applying a light field to the trap and recorded the positions of the trapped atoms using a different laser beam.
Nearly thermalized
By varying the delay between when the caesium atoms are introduced and when they are frozen, the researchers were able to determine how the motions of the caesium atoms change when they collide with the rubidium atoms. They showed that just one collision can strip enough kinetic energy from a caesium atom so that it ends up with almost the same energy as the surrounding rubidium atoms. The caesium atom is thus nearly in thermal equilibrium with the surrounding rubidium atoms after one collision.
Although far from the classical situation in which the Langevin equation applies, Widera’s team discovered that the equation can work under these experimental conditions. However, the equation must first be modified to include a friction coefficient that describes how the viscosity of the medium depends on the velocity of the diffusing atoms.
This modified Langevin equation could be used to describe diffusion that does not involve a continuous medium, say the researchers. Examples are aerosols (mixtures of suspended particles) dispersed in thin layers of air in the upper atmosphere, in interstellar space or in vacuum systems.
The study is described in Physical Review Letters.
- A version of this article first appeared on nanotechweb.org