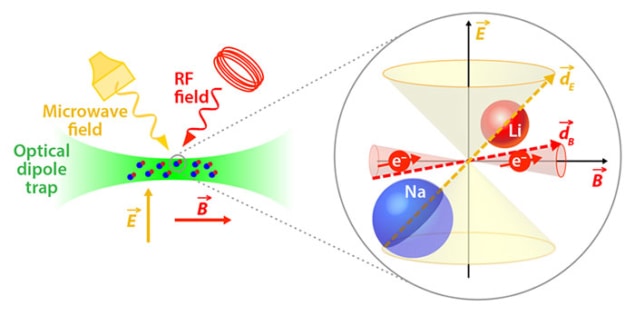
Long-lived, ultracold molecules with both magnetic and electric dipoles have been produced for the first time by researchers in the US. The sodium-lithium molecules have much longer lifetimes than ultracold molecules created previously, allowing the researchers to study them more easily. The system also provides fundamental insights into molecular collisions.
In the past 20–30 years, scientists have become extremely adept at cooling clouds of atoms to nanokelvin temperatures and observing the strange and wonderful physics that occurs. Molecules, however, are trickier to cool as energy has to be removed from many more degrees of freedom such as rotation, vibration and bending. An alternative approach called magnetoassociation has proved more successful. A mixture of the molecule’s constituent atoms is cooled with laser beams before an applied magnetic field is reduced to make the atoms stick together into weakly bound large molecules called Feshbach molecules. These are then further manipulated to make smaller, strongly bound molecules.
Weakly bound
Feshbach molecules are created in the so-called triplet state, which has spin angular momentum and therefore a magnetic-dipole moment. When the technique was used in 2008 to produce the triplet ground state of potassium-rubidium, however, it was so weakly bound that it broke apart within about 170 μs. The researchers therefore redesigned their protocol to produce the lower-energy, zero-angular momentum singlet state. “They said, ‘OK, we transferred to the triplet state, it died immediately: let’s go to the singlet state, which should be much more stable,'” explains Timur Rvachov of the Massachusetts Institute of Technology, who was involved in the new work: “That’s essentially what people have been doing since then, and in most cases their expectations have been correct.”
One exception to the quest for the singlet state was the production of diatomic rubidium molecules in the triplet state by researchers at the University of Innsbruck in Austria, also in 2008. However, as the two atoms were the same, they necessarily had the same electronegativity. The molecule therefore had no electric dipole – although it did have a magnetic dipole. Moreover, the molecules’s lifetime was just over 200 ms.
Bad collisions
The singlet states, being the absolute ground states of the molecules, were originally predicted to have extremely long lifetimes, but recent research has suggested this is not true. Physicists therefore postulate new decay channels dependent not on spin state but on mass: “Heavier molecules tend to undergo bad collisions more often and die more quickly, basically,” says Rvachov.
To study this relationship further, Rvachov and colleagues led by Wolfgang Ketterle – who shared the 2001 Nobel Prize for Physics for the production of a Bose–Einstein condensate using ultracold atoms – produced the triplet state of the lightest possible alkali metal compound with different atoms. They cooled a mixture of sodium-23 and lithium-6 atoms in an optical trap in a magnetic field. When the magnetic field was reduced slightly, it became energetically favourable for the two atoms to form Feshbach molecules. These were then further manipulated by two lasers of precisely defined frequencies in a scheme analogous to those used by the American and Austrian researchers, although the frequencies required are different for each molecule. The researchers measured lifetimes for the molecules of up to 4.6 s. “People are still trying to figure it out but it seems mass is a more important predictor [than spin state] of whether you’ll have a stable sample of molecules,” says Rvachov.
Electron spin resonance spectroscopy
In addition to theoretical insights, the combination of long lifetime and magnetic-dipole moment allowed the researchers to perform electron spin resonance spectroscopy on the molecule. They applied a radio-frequency magnetic field and measured the frequency at which the electron spin flipped. From this, the researchers could infer the hyperfine interaction – the magnetic coupling between the electrons and the nuclei of the sodium and lithium atoms – for the first time.
Florian Schreck of the University of Amsterdam describes the paper as “a great step” towards the larger endeavour of producing a molecular quantum gas, in which all the molecules pile up in the lowest possible energy levels of their trap. He notes that this would require considerable further increases in density and reductions in temperature. Simon Cornish of Durham University in the UK says the most surprising aspect of the research is the long lifetimes of the molecules: “We’ve worked on atomic gases for…well, too long now, and we have a really good understanding of how atoms collide,” he says. “But molecules are much more complicated.”
The research is described in Physical Review Letters.