Since the time of Aristotle, some scientists have claimed that hot water freezes faster than cold. Philip Ball looks at current attempts to shed light on this puzzling phenomenon

It sounds like the kind of question you would be dismayed to hear schoolchildren getting wrong: which takes less time to freeze, cold or hot water? Common sense and the laws of thermodynamics appear to insist that cold water must freeze first. For example, Newton’s law of cooling states that the rate at which a body cools is proportional to the temperature difference between the object and its surroundings. But, in fact, it does seem as though hot water sometimes “overtakes” cold as it cools.
Indeed, Aristotle, Francis Bacon and René Descartes all claimed that hot water does freeze more quickly. Erasto Mpemba, a secondary-school student in Tanzania, may have been unaware of their claims, but it was something he also observed in 1963. To make ice cream for a school project, he was told to boil milk and then let it cool before putting it in the refrigerator. But, fearful of losing his place, Mpemba put his mixture in the fridge while it was still hot. He found that it froze before the other, cooled mixtures.
Others have since claimed to have observed this “Mpemba effect” in their own experiments. Nevertheless, many scientists find it hard to accept such a seemingly counterintuitive phenomenon. The problem is that the effect is frustratingly hard to reproduce – sometimes it appears, and sometimes not. In fact, no-one has agreed exactly how the experiments should be conducted in the first place. And even if the Mpemba effect is real – if hot water can sometimes freeze more quickly than cold – it is not clear whether the explanation would be trivial or illuminating.
Against the grain
Condensed-matter physicist Monwhea Jeng of Southern Illinois University in the US, who has researched the history of the Mpemba effect, believes that scientists are much more likely to react with disbelief than laypeople when they first hear about the phenomenon. That is because scientists know why it “cannot” be right, he says. Indeed, when Mpemba learned about Newton’s law of cooling a few years after making his discovery and asked his teacher how this could be reconciled with his observations, his teacher replied, “All I can say is that is Mpemba’s physics and not the universal physics.”
Fortunately, Mpemba was not deterred by this cruel put-down, and he went on to carry out further experiments of his own. When local physics professor Denis Osborne of University College in Dar es Salaam visited the school, Mpemba seized the chance to ask for an explanation for his findings. Osborne had none, but he was less sceptical than Mpemba’s teacher and wisely concluded that “it is dangerous to pass judgement on what can and cannot be”. Osborne then asked a technician at his university to repeat the experiments, and the results seemed to show that Mpemba was right. In 1969 Osborne wrote about the work with Mpemba (then at the College of African Wildlife Management in Moshi) and published it in the journal Physics Education. Coincidentally, a physicist named George Kell at the National Research Council of Canada in Ottawa reported the same phenomenon that year in the American Journal of Physics.
These reports revealed that the Mpemba effect was already familiar in folk wisdom. Kell, hailing from a country with ample experience of freezing water, stated that “some say that a car should not be washed with hot water because the water will freeze on it more quickly than cold water will, or that a skating rink should be flooded with hot water because it will freeze more quickly”. Mpemba, meanwhile, pointed out that Tanzanian ice-cream makers routinely froze their mixtures while they were hot, because that was a faster method. And when Mpemba’s work was described in an article in New Scientist in 1969, it prompted a rush of anecdotes about food-freezing practices and hot-water pipes freezing while cold ones did not.
Those making such claims are in good company. In his Meteorologica from about 350 BC, Aristotle wrote that “if water has been previously heated, this contributes to the rapidity with which it freezes, for it cools more quickly”. The idea was questioned by the great medieval champion of experimentation Roger Bacon, but his namesake Francis asserted in the 17th century that “water a little warmed is more easily frozen than that which is quite cold”. Francis Bacon was deeply interested in freezing and refrigeration – he is said to have caught a fatal chill while conducting an experiment on preserving a chicken by stuffing it with snow. Around the same time, Descartes made careful observations of the freezing of water that enabled him to identify the liquid’s unusual density maximum at 4 °C. These studies convinced him that “water which has been kept hot for a long time freezes faster than any other sort”.
But were all these reports just the result of bad experimental technique? Surely it should be a simple matter to settle the issue once and for all by carrying out experiments? That turns out to be surprisingly difficult, not least because the statement “hot water freezes faster than cold” is ill-defined. In a recent paper, Jeng suggests a more precise wording (arXiv.org/abs/physics/0512262v1): “There exists a set of initial parameters, and a pair of temperatures, such that given two bodies of water identical in these parameters, and differing only in their temperatures, the hot one will freeze sooner.”
There are many such parameters that could affect the rate of freezing, the most obvious including the volume and type of water used, the size and shape of the containers, and the temperature of the fridge. This presents a significant challenge for the experimentalist, who in principle would have to set up a vast multidimensional array of experiments involving containers with different sizes and shapes, while independently varying the masses and gas content of the water and the refrigeration method used, in order to test for the effect.
There is also the key problem of how to define the time of freezing. Does this refer to the moment when the first ice crystals appear or to the time when the entire body of liquid is frozen? “Both of these times can be very hard to observe, perhaps especially in a refrigerator,” says ice specialist Charles Knight of the National Center for Atmospheric Research in Boulder, Colorado, US.
Looking for clarity
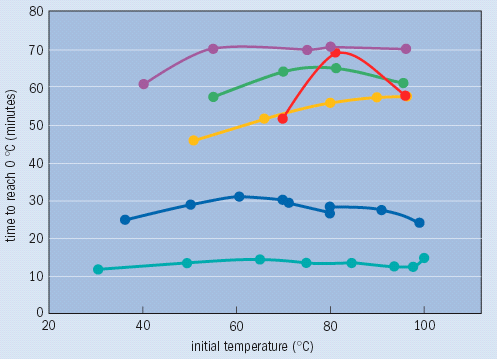
These complexities perhaps explain why the Mpemba effect remains a puzzle to this day. A number of scientists have investigated Mpemba’s claim, but their results remain inconclusive. In 1977, for example, Jearl Walker reported in Scientific American that he had observed the time it took a beaker of water to cool to 0 °C from different initial temperatures under various conditions. These tests provided some clarification of the effect (see figure). But although Walker reported that he could reproduce most of his results, he still obtained large deviations in some of them. “I have not been able to resolve the controversy,” he said.
However, despite the continuing uncertainties surrounding the effect, Pablo Debenedetti, a physicist at Princeton University and a specialist in phase transitions of water, is happy to believe Mpemba’s account. “I do not see any reason to doubt observations showing that under some circumstances hot water can freeze faster than cold water,” he says.
But what causes the effect? Both Debenedetti and Knight point out that there could be at least one obvious explanation for it. If the containers are left open, the hot water will evaporate more quickly and its volume will decline compared with that of the cold water. With a smaller volume, the cooling of the hot water could then overtake that of the cold. That should be easy to test, according to Debenedetti, because the evaporation rate is proportional to the area of the liquid surface. “This can be systematically controlled in experiments conducted in pairs of containers with different geometry,” he says.
Another possibility is that the freezing process could be affected by dissolved gas. Hot water generally holds less dissolved gas than cold, which means that two samples that differ only in their initial temperature would not contain “identical” substances. Debenedetti points out that tiny bubbles of gas can provide nucleation sites where ice crystals start to form. In principle, this might be expected to make ice formation easier in cold water, contrary to the Mpemba effect. But Debenedetti says that the solubility of nonpolar gases such as nitrogen or methane do not necessarily vary smoothly with temperature, so there could be temperature ranges within which the hotter water contains more dissolved gas.
Experiments to pinpoint these influences would require the water to be thoroughly degassed. The effects of other dissolved impurities could be even harder to probe: for example, one could divide the water up into tiny droplets in an oil-water emulsion so that most of them are too small to contain any impurity particles.
Then there is the role of chance, since the nucleation of ice in freezing water depends on enough water molecules coming together to form the core of an ice crystal that can then grow indefinitely. The further the water is below freezing point, the more likely this is to happen. But because it can take some time for ice crystals to nucleate, water can often be “supercooled” such that it remains liquid well below freezing. Random impurities in the liquid, such as specks of dust, can, however, increase the rate of nucleation and suppress supercooling. “Keeping everything constant from experiment to experiment may not be possible without resorting to purposeful nucleation, and that might destroy the effect one is looking for,” says Knight.
Knight adds that he was reminded of the part played by chance while conducting some recent experiments on ice formation. “I had to sit in a cold room at -15 °C and watch water freezing in ice-cube trays on a table top. This exercise emphasized that everything is variable. Some compartments started freezing in about 15 minutes, but many did not for an hour or more.”
Further testing required
In 1995 German physicist David Auerbach at the Max Planck Institute for Fluid Dynamics in Göttingen looked at the role of supercooling in the Mpemba effect. But what he found only made things more complicated. He observed that hot water froze at a higher temperature than cold and therefore in a sense froze “first”. However, the cold water took less time to reach its supercooled state and so seemed to freeze “faster”. To add to the confusion, earlier researchers had reported the opposite: that initially hot water could be supercooled to lower temperatures than cold water. In 1948 Noah Dorsey of the US National Bureau of Standards argued that this is because heating expels impurity particles that acted as nucleation sites for ice. It has been claimed that this effect leads to hot-water pipes bursting more readily than cold, since deeper supercooling leads to ice fingers that advance right across the pipe and block the flow, while freezing nearer to 0 °C just produces a sheath of ice on the pipe surfaces with an open channel in the centre.
Such contradictions continue to make the Mpemba effect as puzzling as ever. Knight is happy to leave it that way, because he thinks that attempts to clarify it would demand too much effort for little return. But Jeng is more positive. He says that despite the complexity of the effect, the experiments needed to study it can be carried out by undergraduates and high-school students – so long as they are planned carefully. As well as thinking about exactly how to heat the water and the kind of thermometer that should be used, experimenters should also consider precise details of the environment surrounding the container. “It can make a difference whether the water is in the middle of an empty freezer, or jammed between a frozen pizza and a frost-covered tub of ice cream,” he says.
Though it is not perhaps the most hi-tech type of experiment, it is one that could help resolve a puzzle that has intrigued scientists for over two millennia. Any takers?