Climate change is a reality we must all face up to, and burning fossil fuels to generate electricity is the biggest contributor. Steve Furnival explains how capturing and burying the carbon dioxide produced could help avert disastrous global warming

The existence of climate change is no longer up for debate. The scientific consensus is that the Earth is warming due to human activity: the level of carbon dioxide – the principal “greenhouse gas” that traps solar radiation in the atmosphere – has risen by more than 50% since the industrial revolution (figure 1). Burning fossil fuels in power stations accounts for about 40% of all human carbon-dioxide generation, but with the insatiable demand for energy and desire for security of supply, many countries are sticking with coal and gas. Indeed, China has plans to build over 500 coal-fired power stations in the next 10 years, 100 more are planned in the US, while India intends to double its electricity-generating capacity by 2015.
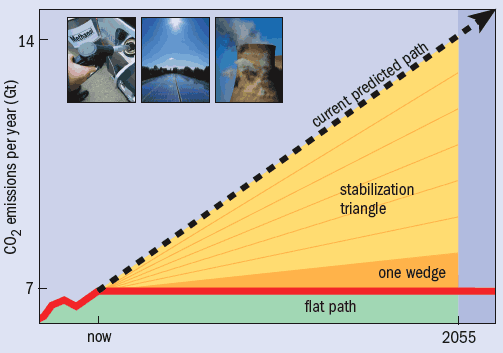
If we continue down this road, humankind’s annual emissions of carbon dioxide (CO2) will double in the next 50 years. But computer simulations show that in order to stabilize the concentration of CO2 in the atmosphere by 2055, and thus prevent climate change from spiralling out of control, we must restrict emissions to the current level of 7 Gt of CO2 per year. Reducing the amount of energy we use is clearly the best solution, and is sure to be increasingly adopted as the price of energy rises. Meanwhile, there is a vigorous debate over the relative merits of renewable sources and nuclear power as ways of producing energy without releasing CO2.
But between reducing the demand for energy and making its supply more “green”, there is a third way. Carbon capture and storage (CCS) is a way of extracting the carbon dioxide generated from the burning of fossil fuels so that it cannot enter the biosphere. This could allow us to continue burning fossil fuels while minimizing the impact on climate change. There is already a working CCS scheme in Norway and a huge worldwide research and development effort in capture and storage technology. Ironically, one of the main commercial incentives for developing CCS is that “waste” carbon dioxide can be used to help extract oil more efficiently from depleted fields.
The ABC of CCS
Carbon capture and storage involves three stages: capturing the carbon dioxide, transporting it, and storing it permanently and safely. The most obvious approach to capturing carbon dioxide is to burn the fuel as normal and then chemically “scrub” the carbon dioxide out of the emissions – a technique that has been used for years in petrochemical plants. To do this the emissions are passed through two reaction towers in sequence: firstly an absorber, which contains droplets of a solvent such as monoethylamine (MEA) in which the CO2 dissolves; then a stripper, in which the MEA is recovered by heating the mixture to release concentrated CO2. The major advantage of this process is it can be retro-fitted to almost all existing fossil-fuel power plants, though at a significant cost.
An alternative method is to capture the carbon dioxide before combustion, by mixing the fossil fuel with steam and air to generate CO2 and hydrogen gas. The hydrogen can be collected and used as a clean fuel, both in power stations and in internal combustion engines – the other main source of greenhouse gases. There is also a third capture scheme whereby the fossil fuel is burnt in the presence of oxygen, producing almost pure CO2 and water, which are easy to separate. However, the higher temperature of combustion in oxygen presents major technical problems.
Once captured, transporting the carbon dioxide is relatively straightforward: we already transport gases in pipelines over thousands of kilometres. As long as any water is removed from it, carbon dioxide poses no particular mechanical or corrosion hazard and is much safer to transport than potentially explosive hydrocarbon gases. Storing the carbon dioxide underground in such a way that little finds its way back to the surface, however, is another matter.
Oil and gas have been trapped underground in hydrocarbon fields for tens of millions of years, so it is reasonable to assume that carbon dioxide injected into fields that we have already depleted will be contained for millennia. But if CCS is widely adopted, these fields will be filled within a few years, so we must also consider alternative underground repositories. By far the greatest capacity is offered by “saline aquifers” – underground reservoirs of water with a high salt content that makes them unsuitable for use as drinking water. The problem is that saline aquifers are not as well understood as hydrocarbon fields.
Encouragingly, we in the oil industry already have considerable experience of storing fluids underground. This is because many countries use depleted hydrocarbon fields and salt caverns to store surplus gas and oil produced in the summer for the high demand in the winter. In the US, for example, the Strategic Petroleum Reserve uses huge salt caverns along the Gulf Coast to store over 700 million barrels of crude oil – worth $50bn at current prices. A similar scheme operates in the UK, using depleted gas fields in the North Sea. Currently, however, European Union (EU) regulations on waste disposal prohibit the burial of carbon dioxide under the sea. But the UK and Norwegian governments are now working with the EU to modify these regulations to allow CCS.
Once a suitable site has been chosen, the mechanics of storing carbon dioxide are not too difficult – it just involves a few pipes, some injection wells and equipment to compress the carbon dioxide before it is stored. The main issue is to ensure that once injected, the carbon dioxide will not find its way back to the surface in any significant amounts. The most likely leak path is through wells, both active and abandoned. Furthermore, when water and carbon dioxide mix they form carbonic acid, so new sealing methods must be developed using cements that are resistant to this chemical attack. Long-term monitoring for leaks will be needed too – a responsibility that must be borne by governments since no commercial organization would take on such an open-ended commitment. While the lifespan of a typical oilfield is between 20 and 50 years, monitoring of CO2 leaks may be needed for millennia.
The main disincentive to wide-scale adoption of CCS is the expense. It is estimated that CCS will cost between $25 and $50 per tonne of CO2, of which 80% is the cost of capture. To get a feel for this, consider that each tonne of coal burned produces about three tonnes of CO2, and that a typical 1 GW coal-fired power station produces 6 million tonnes of CO2 per year. The energy required to operate an effective capture scheme at a power plant would therefore significantly reduce its operating efficiency. Although it should be possible to reduce the cost of CCS by 20–30% in the next decade, further savings will depend on the adoption of the technology together with on-going research and development. In the mean time, a tax on carbon-dioxide emissions would certainly make CCS more economically attractive.
Storage at Sleipner
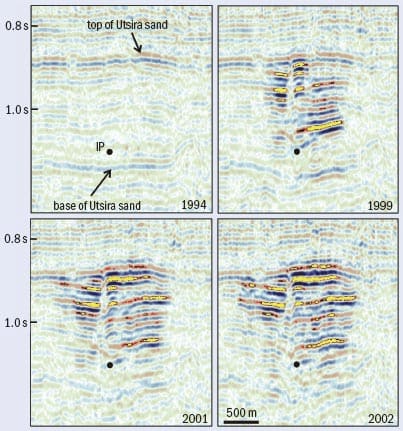
Carbon storage is not just wishful thinking: there is already a successful CCS scheme operating in Norway. The Sleipner gas field was discovered in 1974 and is one of the largest gas producers in the Norwegian sector of the North Sea. However, the gas in the field contains 4–10% carbon dioxide, while typically less than 2.5% is required to ensure the gas will burn properly. In almost any other country, the oil company would have removed the excess carbon dioxide from the gas and vented it into the atmosphere. But under Norway’s environmental laws, Statoil – the state oil company – would have faced an annual carbon-tax bill of about $50m for this option. Instead, Statoil researchers investigated storing the carbon dioxide in a nearby geological formation: the saline aquifer called Utsira that lies above the Sleipner field. Utsira is a massive formation: at some 500 km long, 50 km wide and 200 m thick, it has the capacity to store 100 times the annual volume of carbon dioxide emitted from all Europe’s power stations.
After several years of experimental study, a commercial plant was installed on the Sleipner platform in time for the start of production in 1996. Two MEA absorber columns were installed that reduce the CO2 content of the gas to 2.25%. Four compressors – standard items of equipment on most oil and gas platforms – are then used to pressurize the nearly pure excess carbon dioxide to 80 × 105 Pa, before it is injected into the base of the Utsira aquifer 1 km below. The high pressure is significant because carbon dioxide has a “critical point” at a temperature of 31 °C and a pressure of 74 × 105 Pa, beyond which it exists in a “supercritical fluid” state with a density of about 700 kg m–3. Since injecting CO2 will raise the pressure in the aquifer, the CO2 remains in this fluid state.
Although much denser than a gas, the supercritical CO2 is less dense than water so it will start to migrate upwards. Understanding where and how this fluid moves is the main issue for ensuring long-term capture, and one that is being addressed by teams of geologists, geophysicists and reservoir engineers employed by oil companies to unravel the structure of underground reservoirs.
One method is to generate sound waves at the surface and use them to probe the rock strata beneath. Some fraction of the wave is reflected at the interfaces between the different strata, and the energy received back at the surface can be measured with very sensitive microphones. The resulting data may run to terabytes, and processing it using fast-Fourier-transform techniques requires massive computing power. Indeed, a one-off seismic survey like this typically costs $3–5m, but can only resolve underground objects bigger than about 25 m. By also drilling a test well we can accurately measure a cross-section through the rock strata, which can be input into the computer model to allow researchers to develop a more precise 3D picture of the reservoir.
At Utsira, Statoil has carried out regular seismic surveys in order to reveal the movement of the injected carbon dioxide over time, clearly showing that it travels upwards and spreads laterally (figure 2). The seismic monitoring is supplemented by other measurements, including those of CO2 detectors on the sea floor and measurements of local gravity. Working with researchers at the University of San Diego and the US Department of Energy, Statoil scientists have developed a gravimeter that can monitor changes in the Earth’s gravitational field of 1 part in 200 million, as well as small vertical movements of the sea floor. Additional monitoring is employed at the wells, where leaks are most likely. When the injection of CO2 finally stops, the wells will be plugged using CO2-resistant cements.
A major concern when storing carbon dioxide in saline aquifers is that the natural seal at the top of the formation – a layer of non-porous rock – could be broken during CO2 injection. So far, this seal has remained intact at Utsira, but if it does eventually break, the hope is that a series of shallower seals will minimize the amount of carbon dioxide that will escape. Furthermore, it is believed that over a period of about 1000 years carbon dioxide will dissolve in the brine inside the aquifer, producing a CO2–brine mixture that is heavier than unsaturated brine. The saturated brine will thus move downwards, helping to lock the carbon dioxide away. Longer term still, on geological timescales, it is believed that chemical reactions will turn the CO2–brine mixture into a mineral, locking the carbon dioxide permanently into the Earth’s crust.
Since the Sleipner project began 10 years ago, around 1 Mt of carbon dioxide per year has been injected into Utsira. The initial investment was $80m, most of which was the cost of the capture equipment. Statoil has given no indication of the operating costs, but it is planning a similar project at the Snøhvit field in the far north of the Norwegian Sea. Here the gas produced will be brought onshore via a 143 km pipeline for processing on a small island just outside Hammerfest, from where some 700,000 t of carbon dioxide per year will be sent back to a saline aquifer lying about 60 m below Snøhvit. Statoil is also working together with BP and the Algerian state oil company Sonatrach on a third major project at the In-Salah field in the Algerian desert.
Recovering oil
The Sleipner project only made financial sense because of the Norwegian government’s environmental taxation. However, there is a positive commercial incentive for CCS too: injecting carbon dioxide can help to extract the remaining oil from a flagging oilfield through a process known as enhanced oil recovery. In fact, this procedure, whereby the addition of CO2 makes the oil less viscous, is already used at many oilfields worldwide and much of the carbon dioxide used remains permanently trapped in the pores in the reservoir rock.
Enhanced oil recovery (EOR) is only necessary towards the end of an oilfield’s lifespan. In the initial phase of this lifespan – the “primary” recovery period – high pressure drives fluid into the oil wells and retains hydrocarbon gas dissolved in the oil. This gas swells the volume of the oil and acts as a lubricant, thereby reducing the oil’s viscosity. But as production continues and the pressure falls, the gas bubbles out of solution and the oil becomes more viscous. At this stage, called secondary recovery, water is usually injected to help maintain the reservoir pressure and also act like a piston, pushing the viscous oil ahead of it towards the wells.
As the fraction of water in the fluid rises, so does its density, and hence the pressure needed to get the fluid to flow to the surface increases – requiring the use of pumps, such as the familiar nodding donkeys. Furthermore, the water will eventually find a layer of permeable rock that allows it to bypass the oil and flow quickly between the injectors and wells. The fraction of water in the fluid produced can reach 95% or more, which is separated from the 5% of oil at the surface and then re-injected – an inefficient cycle known as the “washing-machine effect”. A well-managed secondary-recovery scheme can recover 50% of the original oil in the field, but the 50% left behind is a valuable resource, which is where tertiary production, or EOR, comes in.
Carbon-dioxide injection is just one of many EOR methods. Others include using water-soluble polymers that make the injected water more viscous, detergents that reduce the oil’s tendency to adhere to the reservoir rock, or the injection of hot pressurized steam. But for typical crude oils in fields that have previously been flooded with water, carbon-dioxide EOR is usually the best technique. When mixed with depleted oil, CO2 mimics the hydrocarbon gas that was originally dissolved in the oil by re-swelling it and thus reducing its viscosity. Some of the injected carbon dioxide returns to the surface along with the oil, but as CO2 is a major cost of the scheme it is usually stripped out and re-injected.
Carbon-dioxide injection has been widely used for over 30 years throughout the Permian Basin of western Texas and eastern New Mexico, and is responsible for about 15% of the one million barrels of oil per day produced in this region. A network of pipelines over 2000 km long carries the CO2 at a typical cost of $1–2 per tonne per 100 miles with no significant effect on the environment.
As oil prices rise, carbon-dioxide EOR projects are being pursued in many other areas of the US. Most of the carbon dioxide used comes from natural reservoirs of almost pure CO2, but industrial producers are now realizing that their waste CO2 is a valuable resource, valued at $15 to $20 per tonne. For example, carbon dioxide produced at the Great Plains Synfuels coal-gasification plant in North Dakota is now being transported 330 km across the Canadian border, where the oil company EnCana is using it to extend the life of the Weyburn oilfield by 25 years. During this time, the firm hopes the field will produce an additional 130 million barrels of oil and sequester 14 Mt of CO2, equivalent to a year’s emissions from 3.2 million cars.
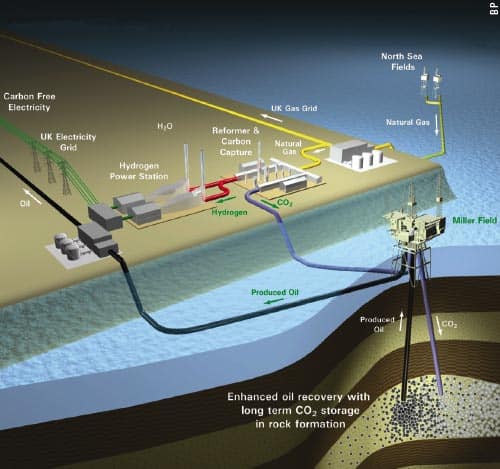
So far, no-one has tried carbon-dioxide EOR offshore, but that will change in the near future. BP is planning a project called Decarbonised Fuel-1 in the UK sector of the North Sea that will combine pre-combustion CO2 capture with EOR (figure 3). North Sea gas will be converted to hydrogen and CO2, with the hydrogen to be burnt in a power station on the Aberdeenshire coast and the CO2 sent offshore to be used to recover oil from the near-depleted Miller field. The plan is to begin injection around the end of this decade for a period of about 20 years.
Meanwhile, Statoil and Shell have announced plans to use CO2 in the Norwegian Sea. Statoil has long been refused permission to build a gas-fired power station in Norway because its emissions would break the government’s Kyoto commitments. By employing CCS in the form of EOR, Statoil can now go ahead and build the plant. If the EU carbon-trading initiative – a scheme that caps nations’ carbon emissions but allows them to buy unused credit from other countries – is given real financial teeth, this may kick-start a similar set of projects across the continent.
The thin end of the wedge
Carbon capture and storage is an extremely active area for researchers from academia, government and industry. Chemical engineers and materials scientists are working to improve capture techniques and reduce their cost; while geologists, geophysicists and engineers are investigating storage methods. Speculative research is even being carried out into storing carbon dioxide in the deep oceans as a heavy CO2–brine mixture that would sink to the seabed. Much of the funding for research and development is being provided by governments eager to meet international agreements like Kyoto, while the rest comes from oil companies and power utilities. For the oil companies there is clear short-term financial gain from EOR, but in the long term there could be a whole new business in sequestering other people’s carbon dioxide. Meanwhile, the threat of carbon-emission taxes is the main driver for fossil-fuel-burning power companies.
The possible contribution of CCS to minimizing climate change has recently been put into perspective by the Carbon Mitigation Initiative (CMI) at Princeton University, a joint project between Princeton, BP and Ford aimed at finding solutions to global warming. It has proposed a simple way to visualize the target of maintaining CO2 emissions at 7 Gt per year rather than the increase to 14 Gt per year predicted by 2055. The target is divided into seven “stabilization wedges”, each of which contribute a saving of 1 Gt per year by 2055 (figure 4). Different schemes can then be compared using the number of wedges they would save. For example, using the most efficient lighting, electrical appliances and insulation in all new and existing buildings would save two wedges. On the supply side, a 50-fold increase in wind power would save one wedge, as would tripling current nuclear power production.
In comparison, carbon capture could save a wedge if it was introduced at coal-fired power stations producing 800 GW of power – equivalent to about two-thirds of today’s production. And if the production of hydrogen from coal and gas as a clean fuel takes off as it is expected to, another wedge could be saved by using CCS at the hydrogen-production plants. Putting these options into practice would require a massive expansion in storage – equivalent to 3500 Sleipner projects per wedge saved – but with the US, China and India heading for a huge increase in new coal-fired power stations, CCS might just be the option that could save the planet.
At a Glance: Carbon capture and storage
- The Earth is warming due to humankind’s activity, and the biggest contribution is emissions of carbon dioxide from power stations
- Carbon dioxide emitted by burning fossil fuels can be captured and stored underground, ensuring that it will not reach the atmosphere
- A large-scale carbon capture and storage scheme is already operating in the North Sea, storing millions of tonnes of carbon dioxide per year
- Carbon dioxide is already used to help recover oil from depleted oilfields, providing a commercial incentive for oil companies to adopt carbon capture and storage
- Storing the carbon dioxide from 800 coal-fired power stations would reduce carbon emissions by the same amount as increasing wind power 50-fold
More about: Carbon capture and storage
www.ipcc.ch – Intergovernmental Panel on Climate Change report on “Carbon dioxide capture and storage”
www.princeton.edu/~cmi/resources/stabwedge.htm – introduction to the Carbon Mitigation Initiative’s idea of stabilization wedges