A novel type of molecule that is longer than some kinds of bacteria has been detected by physicists at the University of Stuttgart in Germany. Using a specially designed microscope, the team observed a binding mechanism between a charged ion and a neutral Rydberg atom – that is, an atom with a single, highly excited valence electron. The extent of the bond length in the new molecule is as wide as a few micrometres, which is at least 1000 times larger than in usual molecules.
When two particles combine to make a molecule, they usually do so in one of two ways: by electrostatic attraction between two oppositely charged ions (ionic bond) or by sharing electrons between two neutral atoms (covalent bond). In contrast, the bond observed by the Stuttgart team forms when the electric field of an ion deforms a Rydberg atom, inducing a dipole in which one side of the atom is more negatively charged and the other more positive. Depending on the orientation of the electric dipole, the interaction between the induced dipole of the Rydberg atom and the charge of the ion can be attractive or repulsive.
What’s unusual about this molecule is that the ion’s electric field distorts the atom in such a way that it causes the dipole’s orientation to flip at a particular distance. At shorter distances, the atom and the ion repel, while at larger distances, they attract. The distance at which this dipole flip occurs determines the bond length of the molecule.
A very cold recipe
To make this molecule, the researchers prepared a cloud of rubidium-87 atoms at a temperature of just 20µK, since higher temperatures would risk the thermal energy of the atoms and ions overcoming the weak strength of the bond. The team then used laser pulses to prepare the molecule’s constituents: firstly ionizing single atoms, then exciting a nearby rubidium atom in the ultracold cloud to the Rydberg state. The Rydberg atom is 1000 times larger than the ion since the more excited the electron is, the farther away from the nucleus it extends. When the Rydberg atom and the ion are separated by a distance comparable to the bond length, a molecule forms.
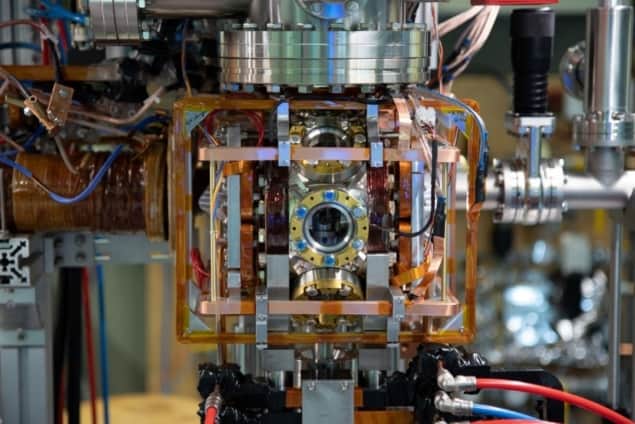
To verify the molecule’s formation, the researchers devised a special ion microscope. Unlike an optical microscope, which uses light to image an object, in this microscope an electric field separates the molecule and ionizes the Rydberg atom. The now separated ion and Rydberg core are then guided along the microscope and onto a detector. Due to their different charge-mass ratios, the Rydberg core and the ion will arrive at this detector at different times, allowing each of them to be detected individually.
The rise of Rydberg physics
Owing to the molecule’s large size, the microscope should be able to measure the motion of the binding partners in the molecule. “The vibrations in this molecule are relatively slow compared to typical molecules and our ion microscope offers enough time resolution to resolve such processes” explains Nicolas Zuber, lead author of a paper in Nature outlining the results. Zuber adds that in the longer term, the ion microscope could also be used to study the dynamics of Bose-Einstein condensates (BECs), which are gases of cooled atoms all occupying the same quantum ground state. Atoms in a BEC behave like a single macroscopic matter wave that extends across the ensemble, and the spatial resolution of the ion microscope is high enough to probe phenomena on a scale similar to the length at which the matter wave changes. It could therefore make it possible to perform spatially-resolved experiments on these quantum gases, for example studies of ionic impurities and ion-atom scattering in the quantum regime.