Pain management is a significant and ongoing health problem. Patients are often treated using opioids, which are effective but also highly addictive. The prevalence of opioid-use disorder and deaths due to overdose has motivated the development of non-opioid alternatives. Of these, nerve cooling could provide an effective and reversible strategy to alleviate pain.
Cooling a nerve causes the pain signals that travel through it to slow down and eventually stop completely. But existing nerve-cooling devices, which use precooled liquids, are bulky, provide non-specific cooling and require high power, making them unsuitable for clinical use. As an alternative, a research team headed up at Northwestern University has developed a soft, flexible implant to cool nerves and provide targeted, on-demand pain relief without the use of drugs, reporting their findings in Science.
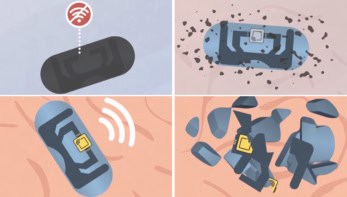
The paper-thin device, which is just 5 mm at its widest point, is constructed from elastomeric materials with tissue-like mechanical properties. As such, it can easily wrap around a single nerve like a cuff electrode to provide effective heat transfer. Another important innovation is that the materials are all bioresorbable and naturally absorb into the body over the course of days or weeks, eliminating the need for surgical extraction and its associated risks.
To create the cooling effect, the implant uses evaporative microfluidic cooling. It incorporates a microfluidic system with one channel containing perfluoropentane, a bioinert liquid coolant that’s clinically approved as an ultrasound contrast and for pressurized inhalers, and a second channel containing dry nitrogen. When the liquid and gas flow into a shared serpentine chamber, the perfluoropentane evaporates and generates localized cooling.
Previous research has shown that reducing the temperature of a nerve to 15°C can block the transmission of compound action potentials, while a complete conduction block is achieved at 5°C. If the temperature is too low, however, there’s a risk of nerve damage. To avoid this, the researchers incorporated a temperature sensor in an electronic layer alongside the microfluidic system to provide real-time feedback and control.
“Excessive cooling can damage the nerve and the fragile tissues around it,” explains John Rogers, who led the device’s development. “The duration and temperature of the cooling must therefore be controlled precisely. By monitoring the temperature at the nerve, the flow rates can be adjusted automatically to set a point that blocks pain in a reversible, safe manner. Ongoing work seeks to define the full set of time and temperature thresholds below which the process remains fully reversible.”
In vivo assessment
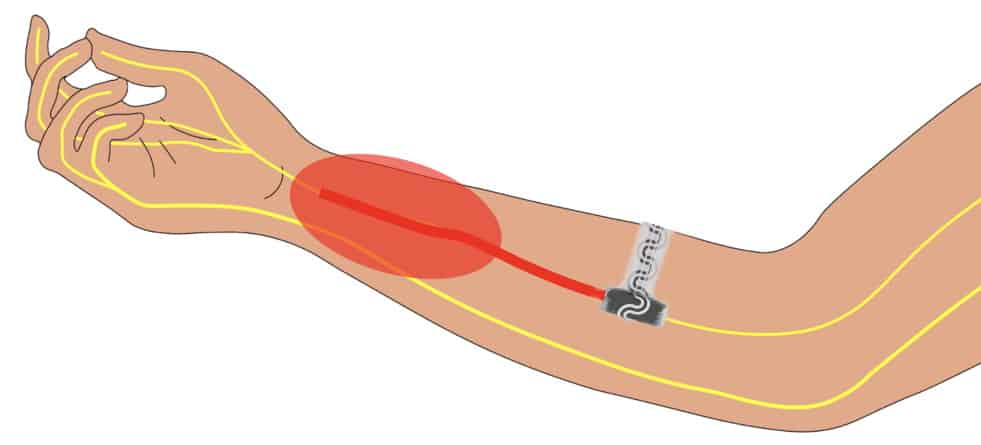
The implant is designed for use on peripheral nerves, which connect the brain and spinal cord to the rest of the body and communicate sensory stimuli, including pain. To demonstrate the device’s cooling ability, the team tested it in rat models of neuropathic pain.
The soft, curled structure interfaced to the rat sciatic nerve without requiring sutures and without causing any damage. The device produced highly localized cooling, which caused effective and reversible conduction blocks of the nerves, as observed by electromyography, compound nerve action potential and muscle-force measurements.

Temporary pacemaker regulates heart rhythm then completely disappears
The researchers also performed experiments in free-moving rats with neuropathic pain over several weeks. When mounted on the animal’s sciatic nerve, the device delivered a significant cooling-induced analgesic effect. They conclude that the cooling device can provide on-demand analgesia to manage neuropathic pain in freely moving animals.
Looking ahead, the team believes that the device could prove most valuable for managing post-operative pain following amputations, nerve grafts or spinal decompression surgeries. In such cases, the relevant nerves are already isolated and identified, making the application of the cuff straightforward to integrate into the clinical workflow.
Writing in a related perspective article, Shan Jiang and Guosong Hong from Stanford University note: “An implantable cooling device with on-demand local analgesia will be a game changer for long-term pain management.”