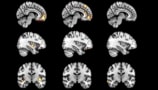
Alzheimer’s disease (AD), the most common cause of dementia, is characterized by progressive cognitive impairment and brain atrophy. Patients are currently assessed using memory and cognitive tests, as well as brain scans that detect biomarkers such as protein deposits in the brain and shrinkage of the hippocampus. But AD remains challenging to diagnose, particularly at early stages of the disease.
A research team headed up at Imperial College London has now demonstrated that a combination of structural MRI and machine learning can diagnose AD from a single brain scan. The technique is based on a predictive model that uses MRI data to identify differences in the brain between people with and without AD. Importantly, the new approach can identify AD at an early stage, even before obvious shrinkage of the brain occurs.
Team leader Eric Aboagye and colleagues developed an algorithm that computes multi-regional features, such as shape, size, intensity and texture, from T1-weighted MRI scans. The model, described in Communications Medicine, uses these features to derive a biomarker called the Alzheimer’s predictive vector (ApV).
The model works by segmenting the MR images into 115 brain regions (45 white matter and 70 subcortical regions) and extracting 656 different features for each region. To avoid overfitting, a least absolute shrinkage and selection operator (LASSO) selects the most informative and least redundant features corresponding to specific brain regions.
To train the algorithm to identify changes that can predict AD, the team used 1.5 T MRI scans obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). This dataset included a control group of healthy individuals, patients with frontotemporal dementia and patients with Parkinson’s disease, plus a disease group of people with AD or AD-related mild cognitive impairment (MCIAD).
The team developed two biomarkers, the first of which – ApV1 – differentiates people with and without Alzheimer’s-related pathologies. To compute ApV1, the algorithm considers features extracted from all brain regions. From these, LASSO selected 20 features in 14 regions and used their weighted sum to determine ApV1. Integrating the model with cognitive measurements and cerebrospinal fluid (CSF)-based biomarkers generated an additional predictive vector, ApV1s.
The researchers tested the model in an unseen internal test set from the 1.5 T ADNI cohort. ApV1 showed an accuracy of 98% in predicting AD-related pathologies. This is more accurate than standard clinical measures – hippocampal volume (26% accuracy) and CSF beta amyloid concentration (62% accuracy) – suggesting a potential alternative to invasive CSF measurements.
They also tested the method on an external test set: 1.5 T MRI data from 64 people in the OASIS consortium. Here, ApV1 and ApV1s exhibited high accuracies of 81% and 83%, respectively.
Disease staging
The second biomarker – ApV2 – categorizes patients with Alzheimer’s-related pathologies into early-stage (MCIAD) and late AD groups. For this classification, a second LASSO used the weighted sum of eight features distributed in seven regions (with a dominance of the left brain) to determine the predictive vector.
In tests on unseen 1.5T data from the ADNI, ApV2 reached an accuracy of 79% in discriminating early-stage from later forms of AD, with a higher accuracy of 86% when integrating cognitive scores and CSF-based biomarkers. This compares with accuracies of 53% and 49%, for hippocampal volume and CSF beta amyloid measurements, respectively. The team notes that this high accuracy is particularly remarkable given the continuum of disease progression between MCIAD and AD.
When the model was applied to 3 T MRI scans, ApV1 and ApV2 showed reduced accuracies of 49% and 62%, respectively. The researchers suggest that the inferior performance at 3 T is likely due to the susceptibility of MRI radiomic features to magnetic field strength, and that this currently limits the algorithm’s use to only 1.5 T data.
The team also tested the model on 83 patients with suspected cognitive decline who underwent clinical amyloid PET imaging at the Imperial Memory Centre as part of their diagnostic workup, along with MRI scans and neuropsychological assessment. PET images were classified as either amyloid-positive, with a clinical diagnosis of AD, or amyloid-negative, likely another type of dementia or a non-neurodegenerative condition.
When employed in this group, the ApV1s outperformed hippocampal volume measurements and standard cognitive scores, showing a statistically significant difference between the amyloid-positive and amyloid-negative groups.
MRI reveals deterioration of brain’s reward circuitry in younger-onset dementia
The researchers conclude that their MRI-based radiomic predictive vector, ApV, is reproducible and robust, can be easily computed and is ready to be integrated into the clinical decision support system. They note that the algorithm identified changes in areas of the brain not previously associated with AD – the cerebellum and the ventral diencephalon – which require further investigation.
“Currently no other simple and widely available methods can predict Alzheimer’s disease with this level of accuracy, so our research is an important step forward,” says Aboagye in a press statement. “Many patients who present with Alzheimer’s at memory clinics do also have other neurological conditions, but even within this group our system could pick out those patients who had Alzheimer’s from those who did not.”