Positron emission tomography (PET) is a medical imaging technology that’s widely used for both clinical and preclinical applications. Within cancer care, the radiotracer 18F-FDG (fluorine-18-fluorodeoxyglucose) is used in PET/CT scans to identify increased glucose intake, a hallmark of cancer cells. And researchers have developed numerous additional radiotracers to target other disease-specific markers.
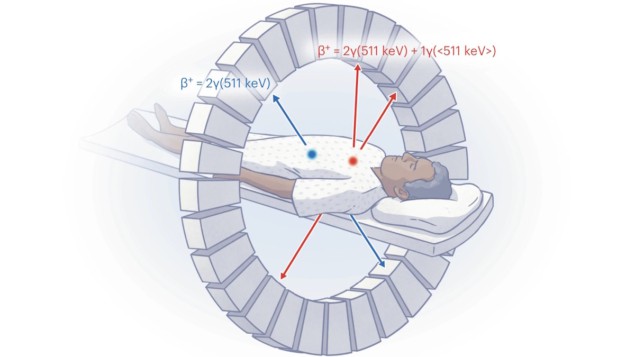
PET works by detecting two 511 keV annihilation photons created when a positron emitted by the radiotracer annihilates with an electron in the body. However, because all PET isotopes produce the same two 511 keV photons, it’s only possible to image one radiotracer at a time. To detect signatures from more than one tracer requires sequential PET scanning, but this is costly, depends on one tracer decaying sufficiently over time and increases the patient’s radiation exposure from the accompanying CT scans.
Addressing this limitation, a research team headed up by Jan Grimm at Memorial Sloan Kettering Cancer Center and Joaquin Herraiz at Complutense University of Madrid has developed a new image reconstruction method that enables in vivo imaging of two different PET tracers simultaneously. The researchers describe their technique, called multiplexed PET (mPET), in Nature Biomedical Engineering.
“This advance could help increase the depth of molecular information attainable during a single scan, giving scientists and radiologists alike more timely information for a diagnosis and staging that could not be done with a biopsy,” explains co-senior author Grimm in a press statement.
Exploiting the prompt gammas
PET images are created using lines of response (LORs) between detector pairs that detect two annihilation photons (“double” events) within a coincidence-timing window of about 3.5 ns. Some positron-emitting isotopes also emit an additional prompt gamma photon. If this is detected within the coincidence window it gives rise to a “triple” event, which is usually considered spurious and not reconstructed. Often, such isotopes are avoided in medical scans.
But first author Edwin Pratt and colleagues have shown how to use this prompt gamma emission to distinguish between two radiotracers in a PET scan. By increasing the coincidence energy window to include the prompt gamma emission, and developing a method for separating and reconstructing double coincidences from triple coincidences, they can generate two separate datasets for each PET scan.
These datasets can be used to produce quantitative images of two PET radiotracers administered simultaneously, with similar performance to two separate acquisitions. “By using a suitable radiotracer pair (one containing a standard positron-emitting isotope and the other an isotope that also emits a prompt gamma), and a proper image reconstruction method (mPET), true simultaneous dual isotope PET imaging can be achieved in most current PET scanners, without modifications or any need for energy discrimination,” says co-senior author Herraiz.
The researchers first tested the feasibility of their mPET method on a preclinical and a clinical PET scanner. They imaged phantoms containing the double-emitting isotope zirconium-89 (89Zr) and the triple-emitting iodine-124 (124I). They found that both systems could acquire data suitable for mPET separation, and that the mPET reconstruction method could create two simultaneous isotope images. They then moved to in vivo preclinical experiments.
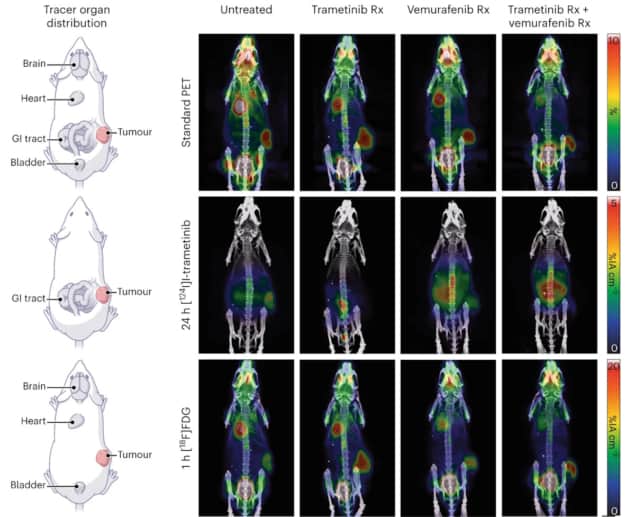
In mice with melanoma tumours, the researchers used mPET to track the biodistributions of two injected radiotracers: 124I-trametinib, which targets proliferating tissue; and 18F-FDG, which targets glucose activity. Upon separating the double and triple events, they observed two distinct biodistributions from the two tracers. They also imaged mice receiving various drug therapies, observing that dual imaging with mPET could be used to track the drugs’ effects on the tumour while also maintaining a standard FDG-PET image.
Tracking drug delivery
Nanoparticles are widely used as drug carriers and as agents to alter drug biodistribution, ideally lowering off-target delivery. Often, such drug delivery is assessed by tracking the radiolabelled nanoparticle and assuming that the drug distribution is the same. But this may not always be the case. To investigate this further, Pratt and colleagues used mPET to noninvasively monitor and quantify a radiolabelled drug and nanoparticle separately.
The team administered 89Zr-ferumoxytol nanoparticles loaded with the cancer drug 124I-trametinib to a melanoma-bearing mouse. Using mPET to separate signals from the drug and the nanoparticle revealed that soon after injection, the drug distribution did not match that of the carrier nanoparticle. This finding suggests that, unexpectedly, most of the drug had dissociated rapidly in vivo, demonstrating a valuable application for this new technique.
The researchers also employed mPET to track CAR T-cells used in immunotherapy to target prostate-specific membrane antigen (PSMA)-positive tumours. In mice with a PSMA-positive tumour, they used 124I to visualize the distribution of CAR T-cells and gallium-68 (68Ga)-PSMA-11 to simultaneously measure PSMA-positive tumour location and expression.
The ability of mPET to separate the two radiotracers showed that there were distinct distributions of 68Ga-PSMA-11 in the tumour, kidneys and bladder, while 124I was found in the tumour, thyroid, kidneys, stomach and bladder. Axial slices through the tumour revealed different intratumoural distributions of the two tracers. The mPET reconstruction provided a way to track the targeting of CAR T-cells while confirming PSMA expression during the same PET scan.

Compact radiation detector could expedite the use of dynamic PET
The researchers conclude that mPET provides additional information via the addition of a second tracer, exploiting many isotopes previously seen as problematic due to their additional gamma emissions. They emphasize that mPET can be implemented on both preclinical and clinical PET/CT systems without any modifications to hardware or image acquisition software. “The beauty is that mPET is immediately clinically translatable,” says Pratt. “The approach can be done on most existing machines, with minimal modifications.”
The next step will be to apply mPET to different studies, the researchers tell Physics World. This includes using mPET to identify cancer resistance to therapy, evaluating it with other positron–gamma emitters, extending its capabilities to imaging more than two isotopes simultaneously, and testing it in the clinic with patients in the right setting.