Soft, implantable optical fibres that move and stretch with the body have been developed by researchers from the US for use in optogenetics studies. The tool will help scientists identify the mechanisms underlying nerve pain and other peripheral nerve disorders in animal models and help to develop new treatments.
Peripheral nerve pain is a condition that occurs when nerves outside of the brain and spinal cord become damaged. Its symptoms can include not only physical pain, but also tingling and numbness in the affected limbs. It is estimated that around 2.4% of people worldwide live with some form of peripheral neuropathy.
When it comes to studying nerve conditions in the brain, scientists can turn to optogenetics. This is a technique in which nerves, mainly in animal models, are genetically engineered to respond to light. By either activating or inhibiting a given nerve, researchers can gain information on how it works and interacts with its surroundings. In fact, optogenetics has already helped to trace the neural pathways underlying a variety of brain disorders, including those affecting mood and sleep, as well as addiction and Parkinson’s disease. The technique has also helped in the development of targeted therapies against these conditions.
To date, optogenetics has largely been confined to the brain, where rigid devices can be implanted relatively painlessly thanks to the lack of pain receptors – and where tissue movement is limited. In contrast, peripheral nerves experience near-constant pulling and pushing from the surrounding muscles and tissues.
As Siyuan Rao, a biomedical engineer at the University of Massachusetts at Amherst, explains in a press statement: “Current devices used to study nerve disorders are made of stiff materials that constrain movement, so we can’t really study spinal cord injury and recovery if pain is involved.” Rigid optogenetic implants also increase the risk of tissue damage. To make optogenetics more practical for nerves located outside of the brain – and potentially also safer within it – Rao and colleagues looked to develop a more flexible form of implant that could move with the body.
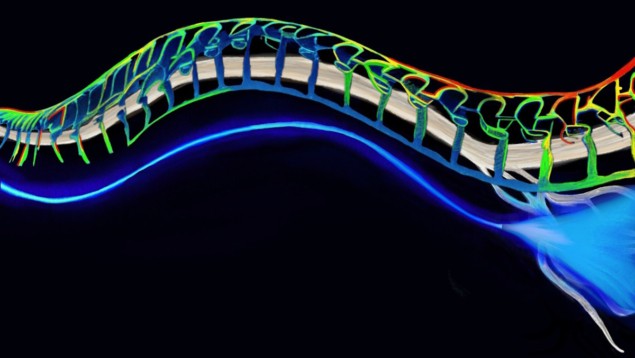
Their solution is a soft, stretchable, transparent fibre made from hydrogel, a biocompatible mix of polymers and water. By fine-tuning the ratio of these ingredients, the team created jelly-like solutions peppered with nanoscale polymer crystals. They used two of these materials – each with a specific refractive index – to fashion the core and outer cladding layers of their optical fibre.
To test the design, the researchers implanted their fibres into mice whose nerves had been genetically modified such that they are activated by blue light and inhibited by yellow light. The team found that the mice were far less sensitive to pain when yellow laser light was sent along the fibre, with such illumination significantly inhibiting sciatic pain in the rodents.
The mice were able to run quite freely on a wheel with the fibres in place, with one end fixed to the skull and the other running down the leg, attached to the sciatic nerve via a flexible cuff structure. Furthermore, the implants remained robust and functional even after two months of running (more than 30,000 deformation cycles), overcoming the limitations of traditional hydrogels.
“Our fibres can adapt to natural motion and do their work while not limiting the motion of the subject. That can give us more precise information,” Rao notes. Co-author Xinyue Liu, now at Michigan State University, adds: “Now, people have a tool to study the diseases related to the peripheral nervous system, in very dynamic, natural and unconstrained conditions.”
“Light serves as a versatile tool for in vivo sensing, imaging and biomodulation. However, the significant optical attenuation in tissues has limited its applicability in various scenarios,” comments Seok-Hyun Andy Yun, a bio-optics expert from Harvard University who was not involved in the study. “The use of minimally invasive hydrogel fibres shows tremendous potential to overcome this limitation, paving the way for expanded applications of optical techniques in animal studies.”
“The novelty of this approach is the relative ease of use, as well as its efficacy and plasticity of usage, adds neurobiologist Federico Iseppon of University College London. “The limitation I would see in this technology is that, even if it surely is an easier approach than wireless implants for peripheral neuroscience studies, it still retains difficult challenges and requires specific technical expertise both in the production of the fibres and their surgical delivery to the tissues of interest.”

Wireless implant uses optogenetics to control spinal cord activity in mice
With their initial study complete, the researchers are now working to scale up their fibres for use in larger animals, and to couple optogenetic control of nerves with the ability to record neural activity.
“We are focusing on the fibre as a new neuroscience technology. We hope to help dissect mechanisms underlying pain in the peripheral nervous system,” Liu added. She concluded: “With time, our technology may help identify novel mechanistic therapies for chronic pain and other debilitating conditions such as nerve degeneration or injury.”
In future, the team says, it may also be possible to use the same approach beyond peripheral nerves, targeting mobile organs like the heart and the gastrointestinal system.
The study is described in Nature Methods.