Traumatic brain injury (TBI), caused by a sudden jolt or impact to the head, requires diagnosis as quickly as possible. To prevent irreversible damage, life-critical treatment decisions must be made within the “golden hour” after trauma. Diagnosing TBI at the point-of-care is difficult, however, relying on observations by ambulance crews followed by radiological investigations such as MRI or CT scans upon arrival at a hospital.
To enable more timely intervention, researchers from the University of Birmingham are developing a handheld diagnostic device that detects TBI by shining a safe laser into the eye. The device, described in Science Advances, is targeted for use as soon as an injury occurs – whether at the roadside, on the battlefield or on the sports pitch – to assess patients for TBI, determine the severity of the trauma and direct treatment accordingly.
The eye-safe device (EyeD) is based around Raman spectroscopy – an optical technique that uses inelastic scattering of laser light to probe molecular composition. It works by shining a 635 nm class 1 laser onto the cornea. The collimated beam is then focused onto the retina by the eye’s own optics. To target the laser to the region-of-interest, the EyeD system simultaneously performs fundus imaging and spectroscopic analysis using a smartphone camera to visualize the back of the eye.
Raman spectra collected from the retina and optic nerve are analysed for the presence of TBI-specific biochemical changes, using the artificial neural network algorithm SKiNET as a decision support tool. As the retina and optic nerve are so closely linked to the brain, changes to biomarkers after injury will reflect biochemical changes in the brain microenvironment.
“Our device will allow early-diagnosis of TBI by directly assessing acute distress changes in real time in living neuroretinal/optic nerve tissue. It enables us to interrogate central nervous system tissue directly and non-invasively,” explains team leader Pola Goldberg Oppenheimer. “Analysing the neuroretina as a projection of the central nervous system provides a window into brain biochemistry.”
Spectroscopic studies
To test the performance of their imaging device, Oppenheimer and colleagues constructed a tissue phantom that mimics the physical dimensions and optical characteristics of the eye, while providing a realistic Raman signature of the retina. The phantom includes a lens, a 4-mm diameter pinhole representing the undilated pupil and sample holder for retinal tissue.
The team demonstrated that the EyeD device could effectively focus the laser beam at the desired position on the retina. Spectra measured from the tissue phantom resolved the major Raman bands in the high-wavenumber region, which can be used to distinguish a number of tissue types.
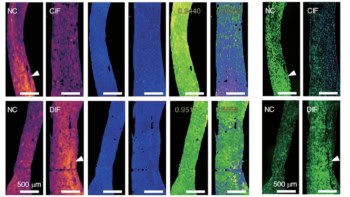
The researchers next used the prototype device to analyse retinal samples from pigs’ eyes, which are similar to human eyes in size, structure, development and composition. They collected 510 measurements from 39 TBI retinal samples and 12 control samples, recording spectra from close to the optic disc. Overall, the Raman spectra showed several characteristic bands in the 1200–1700 cm−1 fingerprint region, plus an enhancement of high-wavenumber bands in the 2800–3200 cm−1 region.
Using SKiNET to create self-optimizing maps (SOMs) showing clustering of the retinal Raman spectra revealed a clear separation between retinas with TBI and control samples. This arises because the Raman spectra reflect biochemical variations in the eye after TBI. For example, TBI increases the lipid and protein content in the eye, causing the peaks originating from these to become more pronounced in the Raman spectra.
The most significant spectral changes in response to TBI were due to the contributions of the brain lipids cardiolipin and cytochrome C, which manifested as an increase in the ratio of the 2930 to the 2850 cm−1 peak in the Raman spectra. The researchers used selected features of the 2850/2930 peak ratio and intensities of six characteristic peaks from the TBI spectra to form the SKiNET classification, yielding a spectroscopic barcode for TBI detection.
To assess the EyeD system’s ability to differentiate TBI via retinal changes, they calculated the area under the curve (AUC) for each peak and the 2930/2850 peak ratio, and plotted true-positive against false-negative rates. Using the SKiNET optimization with 10-fold cross-validation on the training data resulted in a classification accuracy of 90.7±0.9%. This result indicates that changes in the 2930/2850 peak ratio following TBI could provide a valuable indicator to discriminate TBI from healthy controls.
“Using simultaneous Raman spectroscopy and fundus imaging, packaged as a low-cost, handheld device, provides the first tangible path towards non-invasive point-of-care diagnostics of TBI,” Oppenheimer tells Physics World.

For concussions, the eyes are windows to the brain
The next step will be to optimize the prototype for clinical validation. To ease clinical translation, the researchers plan to replace the standalone spectrometer with a compact on-device spectrometer and smartphone readout, enabling fundus photography and Raman spectroscopy via a single smartphone screen.
“We are currently engineering a user-friendly deployable device, integrated with our artificial neural network algorithm for automated interpretation of outputs without requiring specialist support, rapidly classifying spectral data,” says Oppenheimer. “[We are also] clinically evaluating device usability in healthy volunteers and in patients to demonstrate its potential for real-time diagnosis. After establishing device tolerability and usability, we are proceeding to a first-in-human evaluation and small-scale clinical trial.”