A “living contrast agent” could help diagnose mild traumatic brain injury (TBI) when conventional magnetic resonance imaging (MRI) doesn’t show structural changes, say researchers at Harvard University’s School of Engineering and Applied Sciences.
The researchers loaded gadolinium, a standard MRI contrast agent, into hydrogel-based micropatches that attach to immune cells, and in preclinical studies visualized inflammation in pigs with mild TBI. Ultimately, they anticipate that the technology will increase the number of diagnosed mild TBI cases and improve patient care.
“If somebody falls or has a mild head impact, there may not be a detectable change in brain structure, but the brain might still have suffered significant damage which can manifest over time. Suspected TBI patients are told that it looks fine, only to find out that adverse effects show up [later],” says Samir Mitragotri, whose lab conducted the study. “So that was the motivation – can we develop a more sensitive way to detect mild TBI?” The development of the technology was led by Lily Li-Wen Wang, a graduate student in the Mitragotri Lab. MRI expertise was provided by Rebekah Mannix from Boston Children’s Hospital and her team.
Hitchhiking with the immune system’s professional eaters
Since the immune system knows that the brain has been injured, even with “minor” traumas, the researchers sought a contrast agent that could be used to detect immune cells. They homed in on macrophages, white blood cells that are abundant, mobile and, among their other functions in the immune system, are recruited to sites of inflammation and engulf microorganisms.
“Macrophages are notorious for eating whatever binds to them – these are professional eaters,” Mitragotri explains. “We put a label on the macrophage so that the macrophage can be seen on MRI.”
The researchers dubbed the technology macrophage-adhering Gd(III)-loaded anisotropic micropatches, or M-GLAMs. As their name suggests, M-GLAMs attach to macrophages and hitch a ride into the injured brain. Because GLAMs are tagged with gadolinium, the researchers can use MRI to see where the macrophages show up in the brain.
“The macrophage will localize wherever the inflammation is in the brain, so you can see the location of the inflammation. The primary objective, though, is to see if there is inflammation; the secondary question is where, because most of the time in the case of mild TBI, even the first question is not answered,” says Mitragotri.
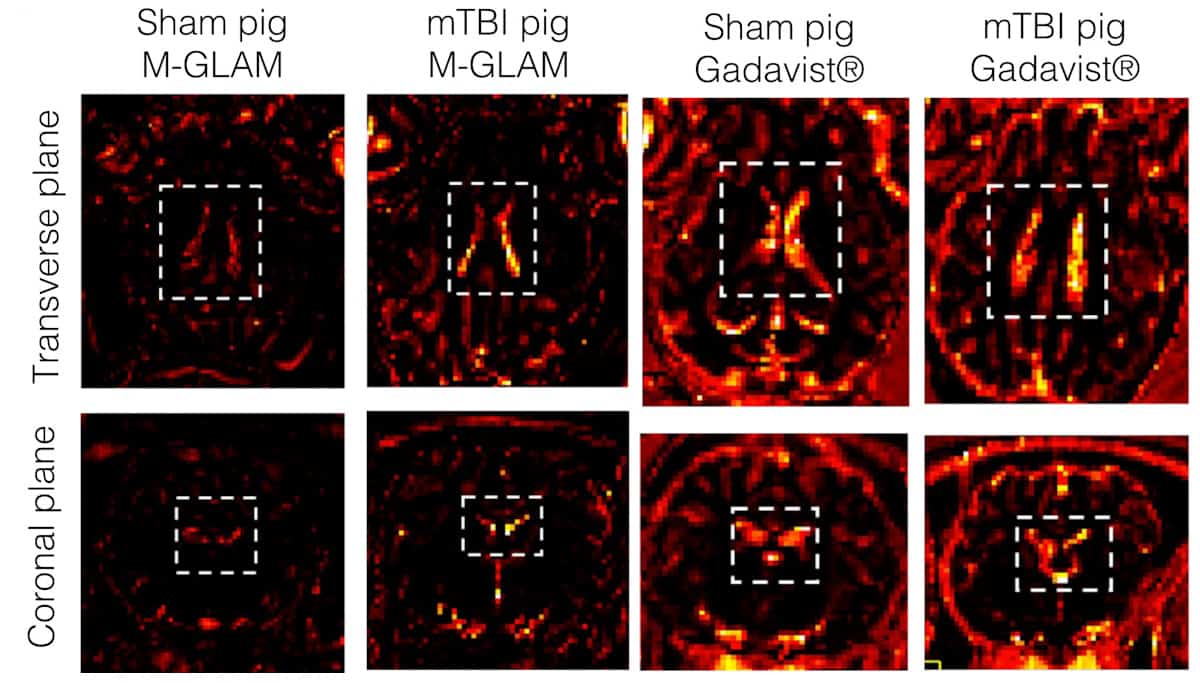
The researchers tested the contrast agent by injecting GLAMs in mice and pigs at a dose of one or more GLAMs per macrophage. Unlike Gadavist, a commercial gadolinium-based contrast agent, M-GLAMs didn’t cause adverse reactions or toxicity and persisted in animals’ bodies for more than 24 h before being cleared by the liver and kidneys. In a porcine brain injury model, they observed M-GLAMs in the choroid plexus, a region of the brain that helps recruit immune cells through the blood–cerebrospinal fluid barrier. Gadavist, which clears from the body rapidly, did not localize to sites of brain inflammation.
The concentration of gadolinium ions in GLAMs is high enough that in animal studies, the researchers were able to use a 500- to 1000-fold lower dose of gadolinium relative to that in Gadavist. They acknowledge that M-GLAMs should be tested in more animals and that M-GLAMs could migrate to sites of inflammation unrelated to mild TBI.
Preparing and characterizing GLAMs
Gadolinium works as an MRI contrast agent where there is contact with water (T1 MRI signals require water proton–Gd(III) interactions). So unlike most polymers used for biomedical applications, which are hydrophobic and non-porous, a GLAM is porous and hydrophilic – a disc-shaped hydrogel that binds to a macrophage when the macrophage tries to eat hyaluronic acid in the hydrogel.
The macrophage fails in this endeavour because the GLAM is disc-shaped (that macrophages cannot eat disc-shaped and other anisotropic particles was discovered by the researchers in the course of another study). Ultimately, GLAMs bind to macrophages without impacting macrophage migration or other functions.
Handheld device uses eye-safe retinal spectroscopy to diagnose brain injury
“The actual process [of fabricating GLAMs] turned out to be quite involved,” says Mitragotri. “Our team worked quite diligently for a few years to get the method of preparation all milled out.” The current fabrication protocol involves mixing modified gadolinium and hyaluronic acid, pouring the liquid into a wafer with wells in it, and spinning the wafer to uniformly fill the moulds. Shining UV light on the spun moulds crosslinks the polymer chains and forms a solid GLAM.
Future work includes detailed kinetic and dose response studies of M-GLAMs in the brain and advancing the technology in humans, where applications include diagnosis and possibly even treatment of mild TBI, cancers and autoimmune conditions.
This research is published in Science Translational Medicine.



