A new class of materials known as “glassy gels” could find use in areas ranging from batteries to adhesives, thanks to their unique set of physical properties.
Meixiang Wang, a post-doctoral fellow from Michael Dickey’s group at North Carolina State University, discovered these new materials while trying out different mixtures for making gels that she hoped would be useful ionic conductors.
Standard gels, such as those used to make contact lenses, are polymers with an added liquid solvent. The liquid weakens the interactions between the chains of molecules forming the polymer, allowing the gel to extend easily but leaving it soft and weak mechanically. In contrast, glassy polymers, like those suitable for airplane windows, contain no liquid and have strong interactions between their constituent polymer chains. This renders them stiff and strong but, in some cases, brittle.
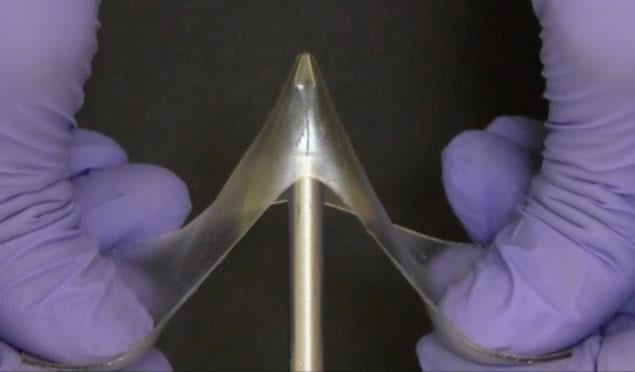
Glassy gels, made by adding liquid solvent to glassy polymers, combine these properties: offering the high stiffness and high strength of glassy polymers alongside high extensibility – they can be stretched to over five times their original length without breaking.
“I thought it was eye-popping when Meixiang told me that these were the toughest gels ever reported by an order of magnitude, and had mechanical properties similar to plexiglass – even though plexiglass has no liquid, whereas these glassy gels are around 60% liquid,” Dickey tells Physics World.
Further tests by the research group, in collaboration with Wen Qian at the University of Nebraska–Lincoln, revealed that the glassy gels also show efficient electrical conduction (Wang’s original aim), good adhesive properties, shape memory characteristics and the ability to self-heal after being cut.
This unusual set of properties, detailed in Nature, is due to the solvent being an ionic liquid (salts in the liquid state). The ionic liquid solvent makes the glassy gels highly stretchable by pushing their polymer chains further apart. But simultaneously, its ions are strongly attracted to charged or polar molecules in the polymer, thereby keeping the polymer chains in place and making the material hard. The solvent ions also conduct electricity, resulting in better conduction than found in common plastics with similar stress–strain characteristics.
While the details of the ion–polymer bonding mechanism are not yet clear, early results indicate that it is electrostatic forces that act over a reasonably large distance. This, Dickey believes, is what makes the materials stiff despite containing so much liquid.
Another plus point for these new materials is their one-step manufacture. The mixture of ionic liquid solvent and liquid precursor of glassy polymers is simply poured into a mould before being cured for five minutes at room temperature with UV light to harden it ready for use.
“In contrast, almost all thermoplastics are made in chemical plants then shipped as resin to factories for melt processing,” says Dickey. He adds that glassy gels could also be 3D printed, and that products made from them would be easier to recycle than those manufactured from multiple plastics, which contain different constituents in order to get the required functionality.

Shear forces help make stretchable hydrogel
In the future, Dickey plans to investigate why glassy gels are so sticky, alongside “tweaking the properties to optimize for particular applications” by changing the ratio of solvent to polymer and using differing types of both constituents. Optimized glassy gels could prove useful as mechanically robust, adhesive and conductive “separators” for keeping the two electrodes in a battery apart, for example, as adhesives, gaskets or seals, or even as heat-driven soft robotic grippers, since the material softens if sufficiently heated.
First, however, Dickey admits that a greater understanding of the gels’ characteristics – including UV stability and degradation over time – is required. But he is encouraged by enquiries from prospective users and optimistic about the potential for this chance discovery by Wang which, as he puts it, “was a bit of serendipity enabled by a researcher who was willing to follow her curiosity”.



