Adaptive radiotherapy – in which a patient’s treatment is regularly replanned throughout their course of therapy – can compensate for uncertainties and anatomical changes and improve the accuracy of radiation delivery. Now, a team at the Paul Scherrer Institute’s Center for Proton Therapy has performed the first clinical implementation of an online daily adaptive proton therapy (DAPT) workflow.
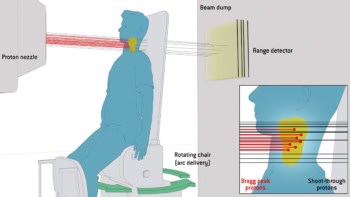
Proton therapy benefits from a well-defined Bragg peak range that enables highly targeted dose delivery to a tumour while minimizing dose to nearby healthy tissues. This precision, however, also makes proton delivery extremely sensitive to anatomical changes along the beam path – arising from variations in mucus, air, muscle or fat in the body – or changes in the tumour’s position and shape.
“For cancer patients who are irradiated with protons, even small changes can have significant effects on the optimal radiation dose,” says first author Francesca Albertini in a press statement.
Online plan adaptation, where the patient remains on the couch during the replanning process, could help address the uncertainties arising from anatomical changes. But while this technique is being introduced into photon-based radiotherapy, daily online adaptation has not yet been applied to proton treatments, where it could prove even more valuable.
To address this shortfall, Albertini and colleagues developed a three-phase DAPT workflow, describing the procedure in Physics in Medicine & Biology. In the pre-treatment phase, two independent plans are created from the patient’s planning CT: a “template plan” that acts as a reference for the online optimized plan, and a “fallback plan” that can be selected on any day as a back-up if necessary.
Next, the online phase involves acquiring a daily CT before each irradiation, while the patient is on the treatment couch. For this, the researchers use an in-room CT-on-rails with a low-dose protocol. They then perform a fully automated re-optimization of the treatment plan based on the daily CT image. If the adapted plan meets the required clinical goals and passes an automated quality assurance (QA) procedure, it is used to treat the patient. If not, the fallback plan is delivered instead.
Finally, in the offline phase, the delivered dose in each fraction is recalculated retrospectively from the log files using a Monte Carlo algorithm. This step enables the team to accurately assess the dose delivered to the patient each day.
First clinical implementation
The researchers employed their DAPT protocol in five adults with tumours in rigid body regions, such as the brain or skull base. As this study was designed to demonstrate proof-of-principle and ensure clinical safety, they specified some additional constraints: only the last few consecutive fractions of each patient’s treatment course were delivered using DAPT; the plans used standard field arrangements and safety margins; and the template and fallback plans were kept the same.
“It’s important to note that these criteria are not optimized to fully exploit the potential clinical benefits of our approach,” the researchers write. “As our implementation progresses and matures, we anticipate refining these criteria to maximize the clinical advantages offered by DAPT.”
Across the five patients, the team performed DAPT for 26 treatment fractions. In 22 of these, the online adapted plans were chosen for delivery. In three fractions, the fallback plan was chosen due to a marginal dose increase to a critical structure, while for one fraction, the fallback plan was utilized due to a miscommunication. The team emphasize that all of the adapted plans passed the online QA steps and all agreed well with the log file-based dose calculations.
The daily adapted plans provided target coverage to within 1.1% of the planned dose and, in 92% of fractions, exhibited improved dose metrics to the targets and/or organs-at-risk (OARs). The researchers observed that a non-DAPT delivery (using the fallback plan) could have significantly increased the maximum dose to both the target and OARs. For one patient, this would have increased the dose to their brainstem by up to 10%. In contrast, the DAPT approach ensured that the OAR doses remained within the 5% threshold for all fractions.
Albertini emphasizes, however, that the main aim of this feasibility study was not to demonstrate superior plan quality with DAPT, but rather to establish that it could be implemented safely and efficiently. “The observed decrease in maximum dose to some OARs was a bonus and reinforces the potential benefits of adaptive strategies,” she tells Physics World.
Importantly, the DAPT process took just a few minutes longer than a non-adaptive session, averaging just above 23 min per fraction (including plan adaptation and assessment of clinical goals). Keeping the adaptive treatment within the typical 30-min time slot allocated for a proton therapy fraction is essential to maintain the patient workflow.
AI framework overcomes segmentation challenges for online adaptive radiotherapy
To reduce the time requirement, the team automated key workflow components, including the independent dose calculations. “Once registration between the daily and reference images is completed, all subsequent steps are automatically processed in the background, while the users are evaluating the daily structure and plan,” Albertini explains. “Once the plan is approved, all the QA has already been performed and the plan is ready to be delivered.
Following on from this first-in-patient demonstration, the researchers now plan to use DAPT to deliver full treatments (all fractions), as well as to enable margin reduction and potentially employ more conformal beam angles. “We are currently focused on transitioning our workflow to a commercial treatment planning system and enhancing it to incorporate deformable anatomy considerations,” says Albertini.