At high temperatures and pressures, molten carbon has two options. It can crystallize into diamond and become one of the world’s most valuable substances. Alternatively, it can crystallize into graphite, which is industrially useful but somewhat less exciting.
Researchers in the US have now discovered what causes molten carbon to “choose” one crystalline form over the other. Their findings, which are based on sophisticated simulations that use machine learning to predict molecular behaviour, have implications for several fields, including geology, nuclear fusion and quantum computing as well as industrial diamond production.
Monitoring crystallization in molten carbon is challenging because the process is rapid and occurs under conditions that are hard to produce in a laboratory. When scientists have tried to study this region of carbon’s phase diagram using high pressure flash heating, their experiments have produced conflicting results.
A better understanding of phase changes near the crystallization point could bring substantial benefits. Liquid-phase carbon is a known intermediate in the synthesis of artificial diamonds, nanodiamonds and the nitrogen-vacancy-doped diamonds used in quantum computing. The presence of diamond in natural minerals can also shed light on tectonic processes in Earth-like planets and the deep-Earth carbon cycle.
Crystallization process can be monitored in detail
In the new work, a team led by chemist Davide Donadio of the University of California, Davis used machine-learning-accelerated, quantum-accurate molecular dynamics simulations to model how diamond and graphite form as liquid carbon cools from 5000 to 3000 K at pressures ranging from 5 to 30 GPa. While such extreme conditions can be created using laser heating, Donadio notes that doing so requires highly specialized equipment. Simulations also provide a level of control over conditions and an ability to monitor the crystallization process at the atomic scale that would be difficult, if not impossible, to achieve experimentally.
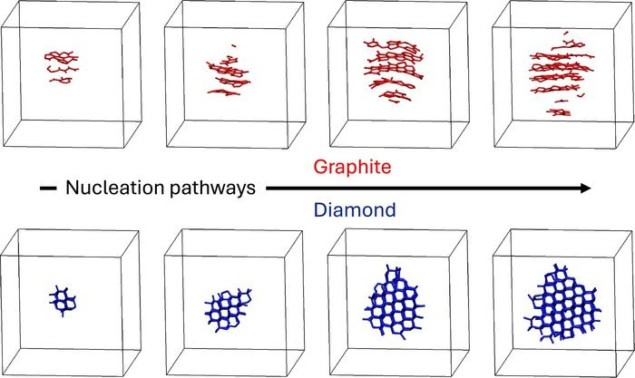
The team’s simulations showed that the crystallization behaviour of molten carbon is more complex than previously thought. While it crystallizes into diamond at higher pressures, at lower pressures (up to 15 GPa) it forms graphite instead. This was surprising, the researchers say, because even at these slightly lower pressures, the material’s most thermodynamically stable phase ought to be diamond rather than graphite.
“Nature taking the path of least resistance”
The team attributes this unexpected behaviour to an empirical observation known as Ostwald’s step rule, which states that crystallization often proceeds through intermediate metastable phases rather than directly to the phase that is most thermodynamically stable. In this case, the researchers say that graphite, a nucleating metastable crystal, acts as a stepping stone because its structure more closely resembles that of the parent liquid carbon. For this reason, it hinders the direct formation of the stable diamond phase.
“The liquid carbon essentially finds it easier to become graphite first, even though diamond is ultimately more stable under these conditions,” says co-author Tianshu Li, a professor of civil and environmental engineering at George Washington University. “It’s nature taking the path of least resistance.”
The insights gleaned from this work, which is described in Nature Communications, could help resolve inconsistencies among historical electrical and laser flash-heating experiments, Donadio says. Though these experiments were aimed at resolving the phase diagram of carbon near the graphite-diamond-liquid triple point, various experimental details and recrystallization conditions may have meant that their systems instead became “trapped” in metastable graphitic configurations. Understanding how this happens could prove useful for manufacturing carbon-based materials such as synthetic diamonds and nanodiamonds at high pressure and temperature.
Liquid carbon reveals its secrets
“I have been studying crystal nucleation for 20 years and have always been intrigued by the behaviour of carbon,” Donadio tells Physics World. “Studies based on so-called empirical potentials have been typically unreliable in this context and ab initio density functional theory-based calculations are too slow. Machine learning potentials allow us to overcome these issues, having the right combination of accuracy and computational speed.”
Looking to the future, Donadio says he and his colleagues aim to study more complex chemical compositions. “We will also be focusing on targeted pressures and temperatures, the likes of which are found in the interiors of giant planets in our solar system.”