It was 100 years ago when Max Planck published a paper that gave birth to quantum mechanics – or so the story goes. History reveals, however, that Planck did not immediately realize the consequences of his work and became a revolutionary against his will.

According to the standard story, which is unfortunately still found in many physics textbooks, quantum theory emerged when it was realized that classical physics predicts an energy distribution for black-body radiation that disagrees violently with that found experimentally. In the late 1890s, so the story continues, the German physicist Wilhelm Wien developed an expression that corresponded reasonably well with experiment – but had no theoretical foundation. When Lord Rayleigh and James Jeans then analysed black-body radiation from the perspective of classical physics, the resulting spectrum differed drastically from both experiment and the Wien law. Faced with this grave anomaly, Max Planck looked for a solution, during the course of which he was forced to introduce the notion of “energy quanta”. With the quantum hypothesis, a perfect match between theory and experiment was obtained. Voila! Quantum theory was born.
The story is a myth, closer to a fairytale than to historical truth. Quantum theory did not owe its origin to any failure of classical physics, but instead to Planck’s profound insight in thermodynamics.
The enigmatic entropy
During the final years of the 19th century, many physicists found themselves discussing the validity of the mechanical world view, which until then had been taken for granted. The question at the heart of the debate was whether time-honoured Newtonian mechanics could still be held as the valid description of all of nature.
In these discussions, which probed the very foundations of physics, electrodynamics and thermodynamics occupied centre stage. As far as the electrodynamicists were concerned, the fundamental problem was the relationship between mechanics and electrodynamics, or between matter and the hypothetical ether. Could the laws of mechanics be reduced to electrodynamics?
Specialists in thermodynamics, meanwhile, focused on the relationship between the laws of mechanics and the two basic laws of heat – the principle of energy conservation and the second law of thermodynamics. This discussion looked at the status of statistical-molecular physics and therefore examined the fundamental question of whether all matter is composed of atoms. Although the two discussions had much in common, it was the latter in particular from which quantum theory emerged.
Max Karl Ernst Ludwig Planck was deeply interested in – even obsessed with – the second law of thermodynamics. According to this law (in one of its many versions), no process is possible in which the only result is the transfer of heat from a colder to a hotter body. With the help of the concept of entropy, introduced by Rudolf Clausius in 1865, the law can be reformulated to state that the entropy of an isolated system always increases or remains constant.
Born in 1858 as the son of a professor of jurisprudence, Planck was appointed professor of physics at the University of Berlin in 1889. His doctoral dissertation from the University of Munich dealt with the second law, which was also the subject of most of his work until about 1905. Planck’s thoughts centred on the concept of entropy and how to understand “irreversibility” on the basis of the absolute validity of the entropy law – the version of the second law of thermodynamics formulated in terms of the entropy concept.
In the 1890s the debate about the second law centred on the statistical (or probabilistic) interpretation that Ludwig Boltzmann had originally proposed back in 1872 and expanded in 1877. According to Boltzmann’s molecular-mechanical interpretation, the entropy of a system is the collective result of molecular motions. The second law is valid only in a statistical sense. Boltzmann’s theory, which presupposed the existence of atoms and molecules, was challenged by Wilhelm Ostwald and other “energeticists”, who wanted to free physics from the notion of atoms and base it on energy and related quantities.
What was Planck’s position in this debate? One might expect that he sided with the winners, or those who soon turned out to be the winners – namely Boltzmann and the “atomists”. But this was not the case. Planck’s belief in the absolute validity of the second law made him not only reject Boltzmann’s statistical version of thermodynamics but also doubt the atomic hypothesis on which it rested. As early as 1882, Planck concluded that the atomic conception of matter was irreconcilably opposed to the law of entropy increase. “There will be a fight between these two hypotheses that will cause the life of one of them,” he predicted. As to the outcome of the fight, he wrote that “in spite of the great successes of the atomistic theory in the past, we will finally have to give it up and to decide in favour of the assumption of continuous matter”.
However, Planck’s opposition to atomism waned during the 1890s as he realized the power of the hypothesis and the unification it brought to a variety of physical and chemical phenomena. All the same, his attitude to atomism remained ambiguous and he continued to give priority to macroscopic thermodynamics and ignore Boltzmann’s statistical theory. Indeed, by 1895 he was ready to embark on a major research programme to determine thermodynamic irreversibility in terms of some micro-mechanical or micro-electrodynamical model that did not explicitly involve the atomic hypothesis. The programme not only expressed Planck’s deep interest in the concept of entropy, but also displayed his “aristocratic” attitude to physics: he focused on the fundamental aspects and disregarded more mundane, applied ideas. His fascination with entropy, which was shared by only a handful of other physicists, was not considered to be of central importance or of providing significant results. And yet it did.
Black-body radiation
From the perspective of Planck and his contemporaries, it was natural to seek an explanation of the entropy law in Maxwell’s electrodynamics. After all, Maxwell’s theory was fundamental and was supposed to govern the behaviour of the microscopic oscillators that produced the heat radiation emitted by black bodies. Planck initially believed that he had justified the irreversibility of radiation processes through the lack of time symmetry in Maxwell’s equations – i.e. that the laws of electrodynamics distinguish between past and present, between forward-going and backward-going time. However, in 1897 Boltzmann demolished this argument. Electrodynamics, Boltzmann showed, provides no more an “arrow of time” than mechanics. Planck had to find another way of justifying irreversibility.

The study of black-body radiation had begun in 1859, when Robert Kirchhoff, Planck’s predecessor as professor of physics in Berlin, argued that such radiation was of a fundamental nature. By the 1890s several physicists – experimentalists and theorists – were investigating the spectral distribution of the radiation. Important progress was made in 1896 when Wien found a radiation law that was in convincing agreement with the precise measurements being performed at the Physikalisch-Technische Reichsanstalt in Berlin.
According to Wien, the spectral density, u, – the radiation energy density per unit frequency – depended on the frequency, f, and temperature, T, according to the formula u(f,T) = af3exp(bf/T)-1, where a and b are constants to be determined empirically. However, Wien’s law lacked a satisfactory theoretical foundation and was, for this reason, not acceptable to Planck. It is important to note that Planck’s dissatisfaction was not rooted in Wien’s formula – which he fully accepted – but in Wien’s derivation of it. Planck was not interested in producing an empirically correct law, but in establishing a rigorous derivation of it. In this way, he believed, he would be able to justify the entropy law.
Guided by Boltzmann’s kinetic theory of gases, Planck formulated what he called a “principle of elementary disorder” that did not rely either on mechanics or on electrodynamics. He used it to define the entropy of an ideal oscillator (dipole) but was careful not to identify such oscillators with specific atoms or molecules. In 1899 Planck found an expression for the oscillator entropy from which Wien’s law followed. The law (sometimes referred to as the Wien-Planck law) had now obtained a fundamental status. Planck was satisfied. After all, the law had the additional qualification that it agreed beautifully with measurements. Or so it was thought.
Discrepancy with theory
The harmony between theory and experiment did not last long. To Planck’s consternation, experiments performed in Berlin showed that the Wien-Planck law did not correctly describe the spectrum at very low frequencies. Something had gone wrong, and Planck had to return to his desk to reconsider why the apparently fundamental derivation produced an incorrect result. The problem, it seemed to him, lay in the definition of the oscillator’s entropy.
With a revised expression for the entropy of a single oscillator, Planck obtained a new distribution law that he presented at a meeting of the German Physical Society on 19 October 1900. The spectral distribution was now given as u(f,T) = af3[exp(bf/T) – 1]-1, which approximates Wien’s law at relatively high frequencies. More interestingly, this first version of the famous Planck radiation law also agreed perfectly with the experimental spectrum in the lower-frequency infrared region. Although it included a constant b that Planck believed was fundamental, the subsequent shift from b to h was more than merely a relabelling. Planck’s derivation did not make use of energy quantization and neither did it rely on Boltzmann’s probabilistic interpretation of entropy.
Those developments were to come two months later in “an act of desperation” as Planck later recalled. Before proceeding to this act of desperation, we need to consider the Rayleigh-Jeans law and the so-called “ultraviolet catastrophe”, if only to discard it as historically irrelevant. In June 1900 Rayleigh pointed out that classical mechanics, when applied to the oscillators of a black body, leads to an energy distribution that increases in proportion to the square of the frequency – utterly in conflict with the data. He based his reasoning on the so-called equipartition theorem from which it follows that the average energy of the oscillators making up a black body will be given by kT, where k is Boltzmann’s constant.
Five years later, Rayleigh and Jeans presented what is still known as the Rayleigh-Jeans formula, usually written as u(f,T) = (8 pi f2/c3)kT, where c is the speed of light. The result is an energy density that keeps on increasing as the frequency gets higher and higher, becoming “catastrophic” in the ultraviolet region. In spite of its prominent role in physics textbooks, the formula played no part at all in the earliest phase of quantum theory. Planck did not accept the equipartition theorem as fundamental, and therefore ignored it. Incidentally, neither did Rayleigh and Jeans consider the theorem to be universally valid. The “ultraviolet catastrophe” – a name coined by Paul Ehrenfest in 1911 – only became a matter of discussion in a later phase of quantum theory.
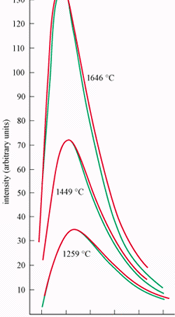
In November 1900 Planck realized that his new entropy expression was scarcely more than an inspired guess. To secure a more fundamental derivation he now turned to Boltzmann’s probabilistic notion of entropy that he had ignored for so long. But although Planck now adopted Boltzmann’s view, he did not fully convert to the Austrian physicist’s thinking. He remained convinced that the entropy law was absolute – and not inherently probabilistic – and therefore reinterpreted Boltzmann’s theory in his own non-probabilistic way. It was during this period that he stated for the first time what has since become known as the “Boltzmann equation” S = k log W, which relates the entropy, S, to the molecular disorder, W.
To find W, Planck had to be able to count the number of ways a given energy can be distributed among a set of oscillators. It was in order to find this counting procedure that Planck, inspired by Boltzmann, introduced what he called “energy elements”, namely the assumption that the total energy of the black-body oscillators, E, is divided into finite portions of energy, epsilon, via a process known as “quantization”. In his seminal paper published in late 1900 and presented to the German Physical Society on 14 December – 100 years ago this month – Planck regarded the energy “as made up of a completely determinate number of finite equal parts, and for this purpose I use the constant of nature h = 6.55 x 10-27 (erg sec)”. Moreover, he continued, “this constant, once multiplied by the common frequency of the resonators, gives the energy element epsilon in ergs, and by division of E by epsilon we get the number P of energy elements to be distributed over the N resonators”.
Quantum theory was born. Or was it? Surely Planck’s constant had appeared, with the same symbol and roughly the same value as used today. But the essence of quantum theory is energy quantization, and it is far from evident that this is what Planck had in mind. As he explained in a letter written in 1931, the introduction of energy quanta in 1900 was “a purely formal assumption and I really did not give it much thought except that no matter what the cost, I must bring about a positive result”. Planck did not emphasize the discrete nature of energy processes and was unconcerned with the detailed behaviour of his abstract oscillators. Far more interesting than the quantum discontinuity (whatever it meant) was the impressive accuracy of the new radiation law and the constants of nature that appeared in it.
A conservative revolutionary
If a revolution occurred in physics in December 1900, nobody seemed to notice it. Planck was no exception, and the importance ascribed to his work is largely a historical reconstruction. Whereas Planck’s radiation law was quickly accepted, what we today consider its conceptual novelty – its basis in energy quantization – was scarcely noticed. Very few physicists expressed any interest in the justification of Planck’s formula, and during the first few years of the 20th century no one considered his results to conflict with the foundations of classical physics. As for Planck himself, he strove hard to keep his theory on the solid ground of the classical physics that he loved so much. Like Copernicus, Planck became a revolutionary against his will.
Planck was the archetype of the classical mind, a noble product of his time and culture. Throughout his distinguished career as a physicist and statesman of science, he maintained that the ultimate goal of science was a unified world picture built on absolute and universal laws of science. He firmly believed that such laws existed and that they reflected the inner mechanisms of nature, an objective reality where human thoughts and passions had no place. The second law of thermodynamics was always his favourite example of how a law of physics could be progressively freed from anthropomorphic associations and turned into a purely objective and universal law. After 1900 he increasingly recognized Boltzmann’s probabilistic law of entropy as grand and fundamental, but he stopped short of accepting its central message, that there is a finite (if exceedingly small) probability that the entropy of an isolated system decreases over time. Only in about 1912 did he give up this last reservation and accepted the truly statistical nature of the second law.

As to the quantum discontinuity – the crucial feature that the energy does not vary continuously, but in “jumps” – he believed for a long time that it was a kind of mathematical hypothesis, an artefact that did not refer to real energy exchanges between matter and radiation. From his point of view, there was no reason to suspect a breakdown of the laws of classical mechanics and electrodynamics. That Planck did not see his theory as a drastic departure from classical physics is also illustrated by his strange silence: between 1901 and 1906 he did not publish anything at all on black-body radiation or quantum theory. Only in about 1908, to a large extent influenced by the penetrating analysis of the Dutch physicist Hendrik Lorentz, did Planck convert to the view that the quantum of action represents an irreducible phenomenon beyond the understanding of classical physics.
Over the next three years Planck became convinced that quantum theory marked the beginning of a new chapter in the history of physics and, in this sense, was of a revolutionary nature. “The hypothesis of quanta will never vanish from the world,” he proudly declared in a lecture of 1911. “I do not believe I am going too far if I express the opinion that with this hypothesis the foundation is laid for the construction of a theory which is someday destined to permeate the swift and delicate events of the molecular world with a new light.”
Einstein: the real founder of quantum theory?
So is December 2000 the right moment to celebrate the centenary of quantum theory? In other words, did Planck really introduce the quantum hypothesis a century ago? The historian and philosopher of science Thomas Kuhn, who carefully analysed Planck’s route to the black-body radiation law and its aftermath, certainly thought Planck does not deserve the credit (see further reading).
However, there is evidence both for and against Kuhn’s controversial interpretation, which has been much discussed by historians of physics. There is a fairly strong case that we ought to wait a few more years before celebrating the quantum centenary. On the other hand, the case can be disputed and it is clearly not unreasonable to chose 2000 as the centenary and Planck as the father of quantum theory. Besides, there is a long tradition of assigning paternity to Planck, who, after all, received the 1918 Nobel Prize for Physics for “his discovery of energy quanta”. Jubilees and similar celebrations enhance traditions, they do not question them.
As Kuhn points out, nowhere in his papers of 1900 and 1901 did Planck clearly write that the energy of a single oscillator can only attain discrete energies according to E = n epsilon= nhf, where n is an integer. If this is what he meant, why didn’t he say so? And if he realized that he had introduced energy quantization – a strange, non-classical concept – why did he remain silent for more than four years? Moreover, in his Lectures on the Theory of Thermal Radiation from 1906, Planck argued for a continuum theory that made no mention of discrete oscillator energy. If he had “seen the light” as early as 1900 – as he later claimed – what caused him to change his mind six years later? Could the answer be that he did not change his mind because he had not seen the light?

These are only some of the arguments put forward by Kuhn and those historians of physics who support his case. Like historical arguments in general, the controversy over the quantum discontinuity rests on a series of evidence and counter-evidence that can only be evaluated qualitatively and as a whole, not determined in the clear-cut manner that we know from physics (or rather from some physics textbooks).
If Planck did not introduce the hypothesis of energy quanta in 1900, who did? Lorentz and even Boltzmann have been mentioned as candidates, but a far stronger case can be made that it was Einstein who first recognized the essence of quantum theory. Einstein’s remarkable contributions to the early phase of quantum theory are well known and beyond dispute. Most famous is his 1905 theory of light quanta (or photons), but he also made important contributions in 1907 on the quantum theory of the specific heats of solids and in 1909 on energy fluctuations.
There is no doubt that the young Einstein saw deeper than Planck, and that Einstein alone recognized that the quantum discontinuity was an essential part of Planck’s theory of black-body radiation. Whether this makes Einstein “the true discoverer of the quantum discontinuity”, as claimed by the French historian of physics Olivier Darrigol, is another matter. What is important is that Planck’s role in the discovery of quantum theory was complex and somewhat ambiguous. To credit him alone with the discovery, as is done in some physics textbooks, is much too simplistic. Other physicists, and Einstein in particular, were crucially involved in the creation of quantum theory. The “discovery” should be seen as an extended process and not as a moment of insight communicated on a particular day in late 1900.
Einstein’s 1907 theory of specific heats was an important element in the process that established quantum theory as a major field of physics. The changed status of quantum theory was recognized institutionally with the first Solvay conference of 1911, on “radiation theory and the quanta”, an event that heralded the take-off phase of quantum theory. The participants in Brussels realized that with quantum theory the course of physics was about to change. Where the development would lead, nobody could tell. For example, it was not believed that quantum theory had anything to do with atomic structure. Two years later, with the advent of Niels Bohr’s atomic theory, quantum theory took a new turn that eventually would lead to quantum mechanics and a new foundation of the physicists’ world picture.
The routes of history are indeed unpredictable.



