Pump–probe microscopy has recently been used to measure the 3D distribution of a pigment in Puccio Capanna’s 14th-century masterpiece The Crucifixion. As Martin Fischer explains, this laser-based technique can give art conservators and historians information that is impossible to get by other means

Museum guards can get very nervous when inquisitive visitors step towards a work of art and lean in for a closer look. After all, someone crazy might want to attack the masterpiece with a knife or spray a load of paint over it. However, for true art lovers, getting right next to a work of art can offer a deeper appreciation of what the artist had in mind. Brushstrokes, colour composition and other detailed features can – once you step back and regard the artwork from its intended viewing distance – almost magically create a wealth of different impressions and emotions.
Quite simply, close-up views can reveal information about the artwork and its artist. Old Master paintings, for example, are very delicately layered objects with many features that are not immediately obvious. Our eyes mostly perceive the paint layers, which are a complex arrangement of coloured pigments, but these are embedded in “binders” that can alter the appearance of the pigments despite themselves being transparent. In fact, some Old Master painters used dozens of glaze layers to achieve the desired colour, gradient and even perceived depth. Leonardo da Vinci’s Mona Lisa is a prime example of such a layering technique, although very few visitors to the Louvre are ever allowed to get close enough to fully appreciate it.
Careful examinations of paintings can also reveal preparatory layers, initial sketches or under-drawings, with the latter being particularly useful to establish the true identity of an unknown artist. Sometimes even entire new art works can be discovered underneath the surface. However, such close-up analyses are best left to professional art conservators and conservation scientists, both equipped with microscopes and other imaging techniques. These people are experts in obtaining highly detailed structural and chemical information, which is crucial in identifying, authenticating, preserving and conserving works of art. Their aim is to identify individual pigments and pigment mixtures, work out how they are layered in 3D and establish how the artist applied them.
Valuable clues about an artwork’s authenticity can also come from checking if the pigments are really those that were used at the time the painting was supposed to have been created and/or seeing if the work has degraded in the manner expected for a painting of that age. Paints, glazes and varnishes all deteriorate naturally when exposed to light and humidity – in fact some early cadmium-yellow and lead-white paints react with each other and gradually turn black with age. Such degradation phenomena can be confined to a few microns of the surface but sometimes they can even encompass entire layers of paint. The challenge for conservators is to identify how much the painting has degraded, what products have been created and what can be done to stop things getting any worse. The specialists have several tools at their disposal, but our group at Duke University, led by Warren Warren, has been working on a new laser-based technique – pump–probe microscopy – that we think could help in that quest.
Tools of the trade
For art conservators, the easiest way to get detailed microscopic information about the composition and layering of pigments is to simply remove a small cross-sectional area of the art work with a scalpel. By laying this piece on its side, the layers can be seen and analysed, just like a sample extracted from an ice sheet or a tissue biopsy in medicine. However, sampling creates spots of damage and, although these marks are almost invisible to the naked eye, this technique should only be used sparingly, particularly on noticeable areas such as someone’s face or with paintings of great value. And even when samples can be taken, they provide information on only a few selected areas of the entire artwork. For some types of art, such as decorated or illustrated manuscripts, sampling is impossible because the pages are much too thin and delicate.

There are, however, some imaging methods that do not leave visible marks on the painting, each having its own strengths and weaknesses. X-rays are a particularly powerful tool, but they can only identify atomic elements and they cannot determine the individual pigments in a mixture. In addition, most X-ray imagers shine radiation through a sample and do not provide information in 3D, although spatially resolved (confocal) versions are being developed that can sample selected points. As for studying larger areas with this technique, that requires access to synchrotron sources, which is costly and not easily obtained.
Cheaper and easier to use than X-rays is light. Infrared reflectography, for example, can reveal under-drawings beneath a whole painting, while ultraviolet fluorescence photography can reveal areas covered with certain varnishes. Selected spots can also be probed with Raman, Fourier-transform-infrared or reflectance spectroscopy, all of which are good at identifying specific pigments, even if they are generally restricted to imaging surfaces, rather than providing 3D information.
In fact, for all mapping tools there is a trade-off between having a high spatial resolution, a large field of view, a good chemical specificity, and the ease (and cost) of use. We think that our new kid on the block – pump–probe microscopy – could find its place in conservation science by circumventing some of these limitations.
The art of being square
To see why pump– probe microscopy can be used in art imaging, it is worth first describing its precursor – a technique developed by physicists that was initially used in the seemingly unrelated field of biomedical imaging. Known as “two-photon fluorescence” (TPF), the technique involves shining infrared light onto a sample containing a fluorescent compound, which emits a photon only if it simultaneously absorbs two separate photons each of roughly half the energy needed to excite the molecule. As not one but two photons need to be absorbed for the molecule to fluoresce, the light emitted is not proportional to the intensity of the incoming light, but instead varies with the square of it. (This nonlinear effect was first predicted by the Nobel-prize-winning physicist Maria Göppert-Mayer back in the 1930s.)
For a physicist, a linear response is generally a good thing, as it often leads to simple, more easily predictable systems. But in optical microscopy, linearity comes with one big drawback. As shown in figure 1a, although the fluorescence is strongest at the focus, it is also generated by out-of-focus light. Even though the fluorescence in these other regions is much dimmer, much of it comes from layers above and below the point to which the light has been focused, making the signal hard to decipher.
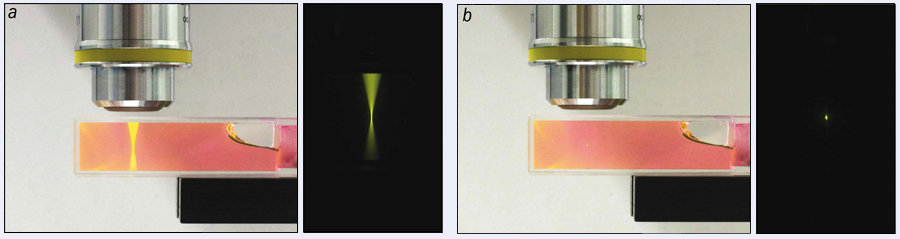
But because the fluorescence scales more strongly with intensity in TPF microscopy than it does in linear techniques, the signal is mostly generated where the light is focused on a sample because here the intensity is at its highest (figure 1b). In fact, for good objective lenses, this region can be as small as 1 μm3.
This localization sounds great, but it comes at a price, which is that nonlinear processes are generally harder to induce than linear ones. In fact, all things being equal, TPF would need a light source about a million times more powerful than those used with ordinary microscopy. But we can make TPF work by turning to ultrafast lasers, which essentially lump a beam’s photons into very short but intense pulses, thereby creating large peak intensities without increasing the average power. Using this approach, in 1990 Watt Webb’s group at Cornell University was the first to implement TPF in a microscope and observe biological samples without damaging them.
Making an object fluoresce while matter surrounding it stays dark is, however, just one way to create the contrast needed to form an image and other groups swiftly developed similar nonlinear mechanisms that can also produce image contrast in biological media. These include second- (or third-) harmonic generation, which involves adding the energy of two (or three) photons to create a single photon of twice (or three times) the energy. Another example is coherent anti-Stokes Raman scattering, in which a photon is inelastically scattered, increasing its energy an amount equal to one of the molecule’s vibrational modes.
Pumping for information
By demonstrating these nonlinear contrast mechanisms, researchers opened the door for nonlinear microscopy to be used to study biological tissue. However, its potential remained largely untapped as it is easier to use techniques that generate light of a different colour to the incoming beam because the emitted light can be detected fairly simply using colour filters. Unfortunately, most nonlinear interactions do not generate such distinct colours and so are harder to detect. While physicists and spectroscopists are familiar with these interactions, current measurement strategies use far too much power to be applicable to tissue (or art).
Nevertheless, some of these other nonlinear techniques can provide the contrast needed to create an image by measuring the intensity of light from different parts of a sample. These include sum frequency absorption (SFA) and excited-state absorption (ESA), both of which involve a sample absorbing two photons of different wavelengths – one from a “pump” laser and the other from a “probe” laser (figure 2). In SFA both photons need to arrive at exactly the same time, while in ESA the molecule first rests in an excited state after absorbing a photon from the pump laser, before only later absorbing a photon from the probe. Another nonlinear process is ground-state depletion (GSD), in which a pump photon excites molecules, with those remaining in the ground state then absorbing a photon from the probe. In stimulated emission (SE), meanwhile, excited molecules provide extra photons that add to the probe beam.
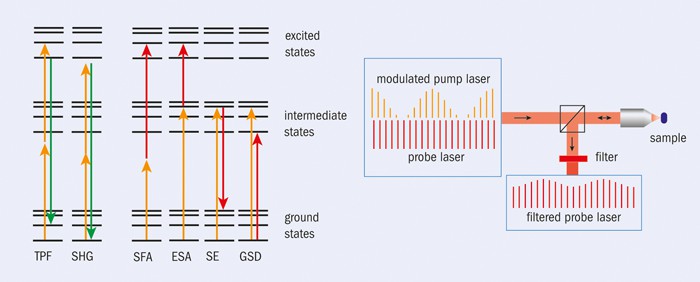
In all of these cases, the nonlinear interaction couples the pump and the probe beam – in other words, turning the pump laser on or off alters the intensity of the probe. So, for example, when the pump beam in an ESA experiment is switched on, the intensity of the probe drops after passing through a sample because the molecule absorbs one photon from both beams. In an SE experiment, in contrast, the intensity of the probe beam rises as an extra probe photon gets generated. And if the incident pump beam is turned on and off periodically, we can use a lock-in amplifier to measure the resulting variation in the probe beam (either from transmitted or back-scattered radiation), with its phase giving information about the type of nonlinear interaction (loss or gain in the probe) and the amplitude relating to the strength of the interaction (or the concentration of a given molecule).
In all cases, this localized excitation can then be used to image the 3D distribution of molecules by scanning the beam across the sample. However, additional details about the molecules can be obtained by introducing a time delay between pump and probe pulse because when the pump pulse excites a molecule, the population in a particular state can relax back to the ground state(s) with a certain time constant that depends on the nature of the molecule. So by switching on the probe pulse a certain time after the pump pulse, we can map out how fast ground-state or excited molecules decay, thereby providing clues as to the composition and structure of the molecule.
Into the art world
Laser spectroscopists have used such modulated pump–probe techniques for decades, but it is only recently, thanks to the development of highly stable, ultrafast dual-colour sources, that these methods have been used to image biological samples. In 2007 our group at Duke was the first to apply this technique to image melanin pigments in skin. Melanin is meant to protect the skin from Sun damage, but in melanoma – an extremely aggressive form of skin cancer – this pigment is involved in uncontrolled cell proliferation. The Duke team has since shown that pump–probe microscopy can provide valuable microscopic information on how this disease develops and spreads.

Although conventional, nonlinear contrast imaging is now widespread in biomedicine, it has been used much less in the art world because the inorganic pigments used in paintings rarely fluoresce. However, our group, which originally developed absorption-based contrasts for biomedical imaging of skin, later realized that these contrasts could also be applied to pigments in artwork. After all, pigments are rather good at absorbing light, which is why they are on a painting in the first place.
Biological tissue and paintings might seem very different objects, but they are in fact rather similar. Both are complex, delicate and microscopically heterogeneous arrangements that contain highly absorbing pigments that need to be identified and localized. In fact, the most obvious way to make a realistic-looking face is to make the paint structure match the absorption and scattering properties of skin – a point that Leonardo da Vinci understood empirically, even if he had no tools to measure those properties.
Armed with a biomedical microscope and tissue-imaging experience, our team therefore set out to test the pump–probe microscope’s capability to image paint pigments in 3D. Collections of paper strips with small paint samples from Kremer Pigments – a company specializing in the manufacture of historical pigments – provided a convenient testing ground for the new microscope application. To our surprise, about half of the tested pigments provided a signal strong enough for imaging, even with a pump and probe wavelength combination that was simply set to image melanin, rather than the pigments.
In fact, we showed that 3D images can be acquired, letting us extract “virtual optical cross-sections” – maps of the distribution of pigments – without optically or mechanically damaging the sample in any way (2012 Optics Letters 37 1310). Figure 3 shows the pump–probe dynamics with a single-depth optical cross-section image for a representative selection of pigments, ranging from organic to mineral, and from synthetic to natural.
A leap of faith
After obtaining contrast in art-pigment samples and demonstrating non-invasive 3D cross-sectioning capabilities in lab-made test samples, the time was ripe for an actual demonstration with real artwork. But where does one get access to relevant historic works of art? Fortunately for our group at Duke, we are only a short drive away from the North Carolina Museum of Art (NCMA), which not only houses a world-class art collection but also has enthusiastic and exceptionally skilled conservators and curators. In what proved to be a truly interdisciplinary collaboration – and with a certain leap of faith! – the conservators agreed to bring Puccio Capanna’s 1330 painting The Crucifixion to our lab and let us test it with our pump–probe microscope. Figure 4 shows this Renaissance master painting undergoing the procedure, from which the painting emerged visibly unscathed.
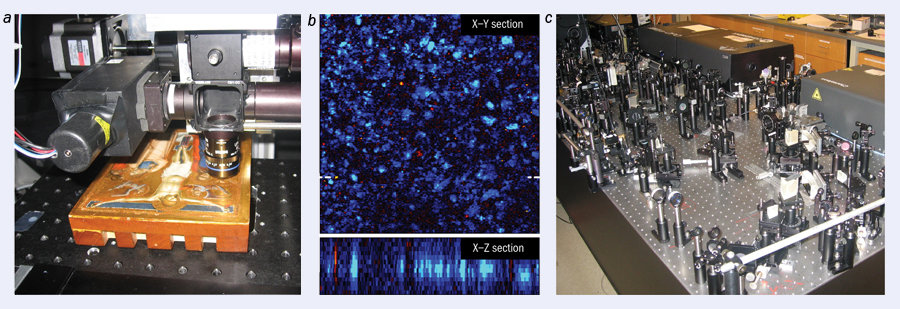
In this demonstration, the lasers were tuned to map lapis lazuli, a precious blue mineral pigment. In the Renaissance age, lapis lazuli – being so rare – was actually more expensive than gold and was therefore used sparingly, primarily for iconic religious figures. Pump–probe microscopy was able to map the distribution of the pigment through the entire paint layer, which in this case was a surprising 60 μm thick, with micron-scale 3D resolution.
Being able to show that our microscope can non-invasively image a painting was naturally exciting. However, the current microscope system is bulky – requiring optics benches and other laser-lab paraphernalia – and was optimized for tissue imaging, rather than for works of art. Much more work remains to be done before pump–probe microscopy can be more widely used in the art world. In particular, the equipment would need to be portable so that it can be taken to conservation labs and easily adjusted so that it can be used to investigate many different parts of an artwork.
Fortunately, the Duke and NCMA collaboration has recently been able to secure funding from the National Science Foundation to bridge the gap between imaging skin and art. Among the aims of our research grant is to develop a much more compact microscopy system, which could provide conservation scientists and conservators with the opportunity to evaluate artworks, including not just painted or coloured works but also pottery and statues. One interesting possibility is to study iron oxide, the pump–probe signature of which seems to change permanently when heated – suggesting that firing history in terracotta could be measured. Nonlinear microscopy could also be used to detect paint fragments buried under the surface (for example, on Greek statues), or even read a delicate scroll without unrolling it.
Much work remains to be done, of course, yet the promise of pump–probe microscopy is genuine. What’s more, it reveals an unusual but fabulous spin-off from society’s investment in basic biomedical research.