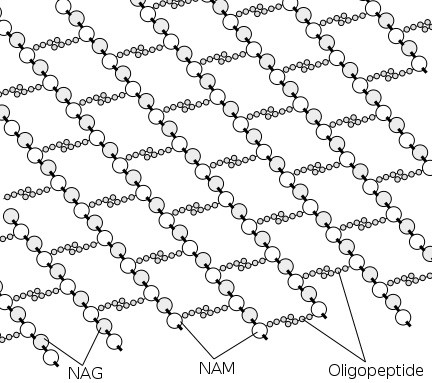
The biopolymer peptidoglycan that makes up bacterial cell walls was always assumed to be highly ordered. Textbook images like the one below have shaped our thinking. Robert Turner and his colleagues have now taken the first high-resolution images of the bacterial cell wall using atomic force microscopy (AFM), revealing that the polymer is much less ordered than previously thought. Additionally, the level of order changes with cell shape: in rod-shaped bacteria, polymer strands are somewhat ordered, while this order disappears when a round cell shape is induced (Nature Communications 10.1038/s41467-018-03551-y).
The team, from the research groups of Simon Foster and Jamie Hobbs at the University of Sheffield, isolated cell wall fragments and imaged them using AFM. This technique can take images at nanoscale resolution. In contrast to the textbook pictures, the images showed that peptidoglycan strands do not run exactly parallel and can even cross over one another. Analysis based on a Fourier Transform calculation showed that there is some order in the polymer, it is just not as high as assumed.
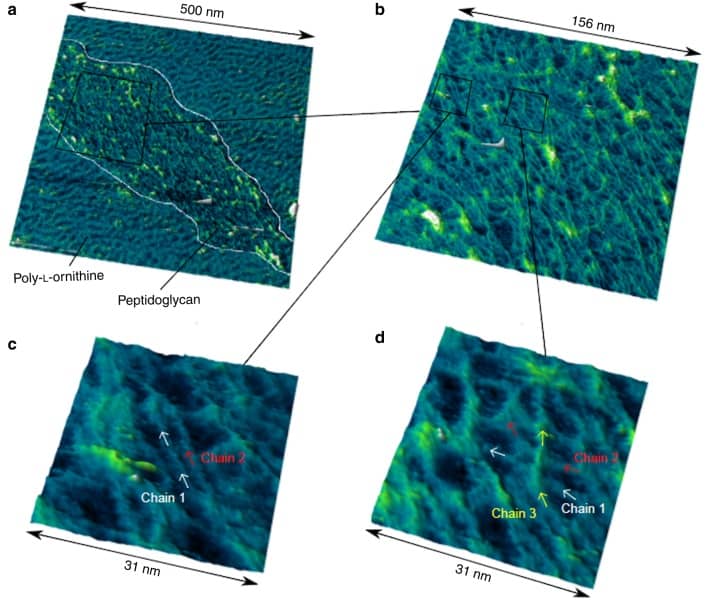
The fact that the polymer is less ordered than originally thought also opens up the possibility of answering another question: “How do bacteria interact with their outer membrane that lies beyond the cell wall?” Turner’s findings show that pores in the polymer could allow molecules to pass through the peptidoglycan wall and connect the inner and outer membranes. Further studies will need to confirm this hypothesis.
A long road to single-chain images
AFM works by recording the movement of a microscopically small cantilever tip scanning over a surface. The resulting image shows the topology of a surface much like the contour map of a mountain. Despite AFM being a powerful technique, Turner and his colleagues had to optimize the process to obtain images that resolve individual polymer strains. “After about a decade of looking at the bacterial cell wall with AFM, I managed to image individual molecular sugar chains,” Turner stated on Twitter.

Round cells are different
The authors investigated the length of polymers using a technique called size exclusion chromatography to separate fragments of different lengths. They found that the rod-shaped bacteria contained long strands of polymers.
When a round shape was induced in the same type of bacteria, by adding chemicals or making genetic changes, the polymer was disordered and contained shorter polymer chains. This might come as a surprise to some who thought that a round cell consisted of two cell poles stuck together. In such a case, the polymer strands would be oriented in a spindle like fashion, which was not observed in Turner’s experiments. It will be interesting to see how peptidoglycan is organized in bacteria that are naturally round.
This works lays the basis for bio-inspired nanostructures and improves knowledge regarding the bacterial cell wall, an important drug target for antibiotics.