
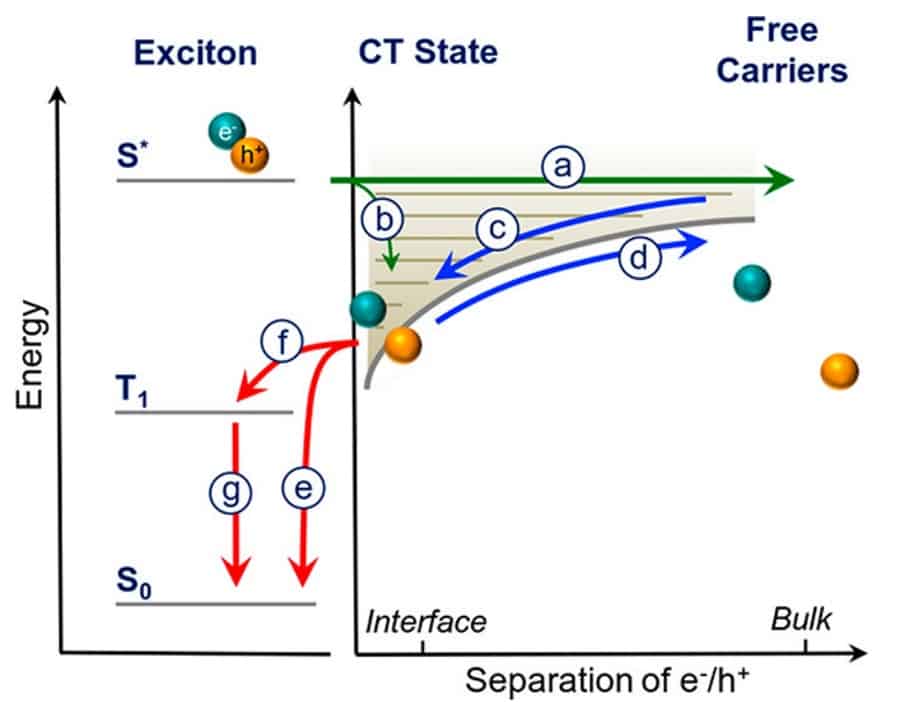
When a photon is absorbed by a solar cell, the first step to producing electricity is to separate the electron and hole pair (exciton) it creates, so that these opposite charges can travel to opposite electrodes. In an organic solar cell (OSC), the charges are physically separated between two materials – one to collect the positively charged holes (the donor) and one to collect the electrons (the acceptor). Optimising charge transport in this material blend is the key to OSC efficiency. In an article published in ACS Nano last month, Yasunari Tamai and coworkers at the University of Cambridge(UK) and the Georgia Institute of Technology (USA) showed that charge separation with a new small-molecule acceptor material (based on perylene diimide, PDI) occurs on the same ultrafast (< 1 ps) timescale as in a state-of-the-art fullerene-derived acceptor, disproving the conventional wisdom that smaller molecules had an efficiency-limiting disadvantage at this initial step.
Breaking the electron-hole pair is only the start of the journey for charge separation in a solar cell: charges have a bumpy ride once separated and can still recombine and decay before they can be captured at their electrodes. Ultrafast spectroscopy enabled the researchers to resolve this journey, identifying the benefits and bottlenecks for charge transport within different OSC materials, and illuminating new pathways to efficient OSC design.
Last year, a different team found that a similar PDI-based small molecule acceptor could achieve fast charge separation, creating a non-fullerene OSC with 9.5% efficiency (reported in Nature Energy). Now Tamai and coworkers have blended either their PDI acceptor or the state-of-the-art fullerene acceptor (PCBM) with a common donor polymer. Despite sacrificing some efficiency, these blends allowed them to directly compare the two acceptor materials, and to confirm that the spread-out electron states of the larger fullerene molecules were not crucial to quickly pull apart the initial exciton pair.
Tracking charge transport
To track the journey of electrons and holes to sub-picosecond resolution, the team used a technique called transient absorption spectroscopy. The technique maps the energy landscape of the polymers by creating excitons with an initial ‘pump’ laser pulse, and then measuring how the absorption has changed with a second pulse at different delay times. Tracking absorption changes across multiple wavelengths, they can extract what electronic states are evolving, over femtosecond to microsecond timescales.
The researchers found the PDI dimer still lagged behind the fullerene-based acceptor on ‘longer’ (nanosecond) timescales. From 1 ns after excitation, the evolution of its absorption suggested electrons in the PDI blend were more likely to meet holes from different exciton pairs and recombine. Electrons also travel 1-2 orders of magnitude more slowly in the PDI blend than in the fullerene acceptor, giving them more time to recombine with holes before extraction.
Tamai and coworkers have signposted ‘later-stage’ recombination and slow electrons, not initial charge separation, as roadblocks for non-fullerene OSCs. This shows the way for scientists to optimize these new small-molecule acceptors, with blends that improve on the 5.9 % efficiency reported here. Their next step is to beat the > 10 % efficiency of the more mature fullerene OSC materials, and eventually to compete with the 20 % efficiency ballpark of commercial silicon technology, as a cheaper, more flexible option for scalable, sustainable solar energy harvesting.



