An international team of scientists has synthesized a carbon nanowire doped with ruthenium and nitrogen that significantly outperforms conventional platinum-based catalysts for producing hydrogen from water. The team attributes the improved catalytic activity to individual ruthenium atoms embedded in the carbon matrix, rather than the presence of ruthenium nanoparticles.
The ability to generate clean and sustainable energy from hydrogen depends on making electrochemical water splitting cheaper and more efficient. Platinum is currently used as the catalyst of choice for water electrolysis, but it is most efficient in acidic conditions – which are impractical for most applications because they demand expensive proton-exchange membranes. In the preferred alkaline electrolytes, however, the catalytic activity of platinum nanoparticles is some two orders of magnitude lower. As a result, hydrogen production by electrolysis is not competitive enough to be widely used and most hydrogen is industrially obtained by steam-reforming methane.
Researchers have therefore been searching for better water-splitting catalysts, with most attention focusing on other noble metals similar to platinum, notably ruthenium. Carbon nanowires doped with ruthenium have shown promise, which has generally been attributed to the presence of ruthenium nanoparticles.
However, while comparable, the performance of these alternative catalysts hadn’t yet surpassed that of commercial platinum versions. But the ruthenium-based catalyst demonstrated by scientists in the US, China and Canada has now set a new efficiency record for water electrolysis.
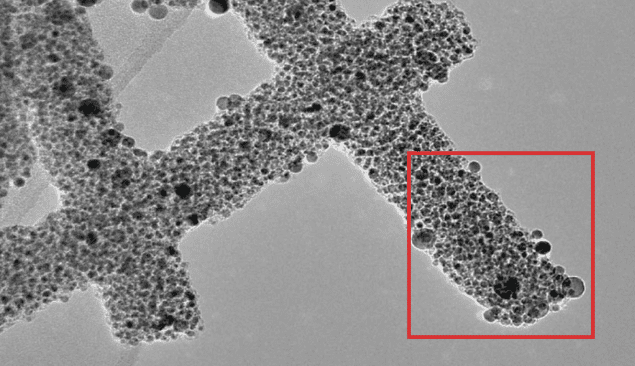
The scientists prepared carbon nanowires co-doped with ruthenium and nitrogen at several different temperatures. In all cases, a transmission electron microscope showed that most elements were uniformly distributed throughout the samples. This included oxygen and tellurium, traces of which were left over from the fabrication process. The one notable exception was ruthenium, which formed dense clumps in the nanowire – confirming that both single atoms and ruthenium nanoparticles were present in the samples.
When the team tested the samples’ water-splitting performances, they found that all of them exhibited catalytic activity – even in a weakly-alkaline environment. Furthermore, the sample prepared at 700 K displayed a very low electron transfer resistance – about 20 Ω, compared with 136 Ω in platinum-based catalysts. This sample outperforms not only current commercial catalysts, but also other recent experiments with ruthenium-based materials.
When the researchers adjusted the fabrication method to reduce the number of nanoparticles in the samples, the results were practically unchanged. This suggests, say the researchers, that the ruthenium nanoparticles are not the main contributor to the water-splitting activity, as was previously thought.
The researchers also ran some simulations to determine which active centres contribute most to the water-splitting activity. They tested different arrangements of ruthenium and nitrogen atoms embedded in the nanowires, taking into account both the energy needed to form these configurations and the energy needed to adsorb a hydrogen atom to the catalyst surface.
Their analysis revealed that the RuC2N2 atomic arrangement is the most active site for hydrogen production. The researchers hope that results will help scientists to design more efficient ruthenium single-atom catalysts, while also highlighting the importance of atomic-scale mechanisms in understanding these materials’ electrocatalytic reactions.
Full results are presented in Nature Communications.