A research team at the Massachusetts Institute of Technology (MIT) has designed medical devices that break down inside the body when they are exposed to light from an LED “pill”. Such an approach would avoid the need for invasive endoscopic procedures that are currently used to retrieve implants and other medical devices when they are no longer required.
“This study is a proof of concept that we can create this kind of material,” says Giovanni Traverso, the study’s lead author, who is also a gastroenterologist at Brigham and Women’s Hospital. “Now we’re thinking about the best applications for it.”
The key to the design is a light-sensitive hydrogel that degrades when irradiated with blue or ultraviolet light. This type of gel is too weak to survive for long inside the body, so the researchers made it more durable by combining it with a stronger polymer network. “You’re forming one polymer network and then forming another polymer network around it, so it’s really entangled,” explains co-author Ritu Raman. “That makes it very tough and stretchy.”
The material’s mechanical properties can be varied by altering the composition of the gel, while the time taken to degrade the material can be tuned by changing the distance between the LED and gel, as well as the intensity and wavelength of light. Ultraviolet light works more quickly, while blue light offers an alternative for cells that might be damaged by higher frequencies.
A balloon inside the stomach
In this study, reported in Science Advances, the MIT team incorporated their hydrogel into a specially designed gastric balloon, a device that is commonly used to limit food intake. The standard approach is to insert the balloon into the stomach through the oesophagus, fill it with saline, and then retrieve it with an endoscope about six months later.
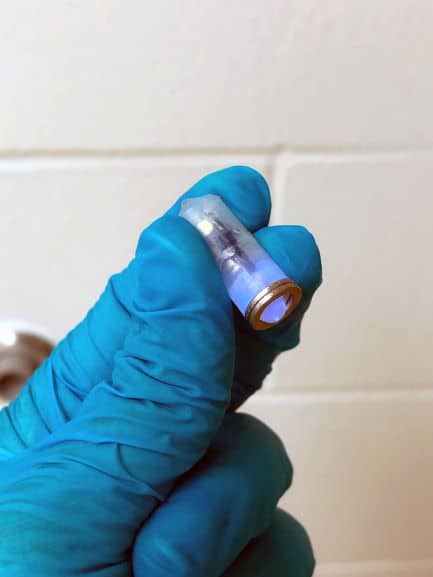
In this case, however, the team fill the balloon with a material that absorbs water, and has a porous outer layer to allow the balloon to swell inside the stomach. They then seal the balloon with the photoactive hydrogel, so that light emitted by a small LED can break the seal to deflate the balloon. Once the LED capsule has been ingested, it is guided to the right location by a magnetic cap that attaches to a metallic piece close to the seal.
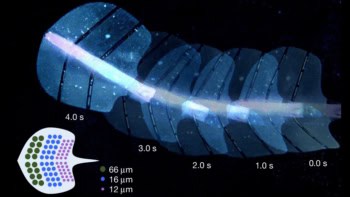
The researchers tested their approach by inserting the balloons into pigs, which have similar-sized stomachs to human adults. Once the balloon had swelled, an LED capsule placed inside the stomach attached itself to the balloon within a few minutes, where it irradiated the hydrogel seal for 70 minutes.
Images taken six hours later revealed that the balloon had deflated as expected, while balloons inserted into control animals had continued to swell. In an alternative setup, an LED array attached to the end of an endoscope was able to break the seal within 30 minutes.

Structural support and drug delivery
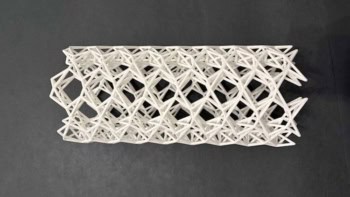
The researchers also exploited a stiffer version of the hydrogel to create a simple stent for the oesophagus, which could be used for structural support or local drug delivery. Preliminary tests showed that a stent inserted into an oesophagus degrades within 30 minutes of being irradiated with an endoscopic LED array, which would allow a similar device inside the body to be dispersed and passed through the digestive tract without harming the surrounding tissue.
Traverso and colleagues are confident that the technique is flexible enough to be used in many other applications. “The material properties, device shape, degradation timeline, and method of delivering light stimuli can all be individually tuned to suit different clinical needs,” he comments.