
Prompt gamma imaging enables real-time beam range measurements during particle therapy, by detecting the prompt gamma rays produced when the treatment beam interacts with atomic nuclei within the patient. Such in vivo range verification should improve the accuracy and effectiveness of proton and ion-beam therapies, which are highly sensitive to even slight targeting inaccuracies.
A recent variant of this technique, prompt gamma spectroscopy (PGS), uses time- and energy-resolved detection of prompt gamma rays for range verification. And because PGS measures the energy of the emitted prompt gammas, it can also be used to determine the elemental concentrations of irradiated tissues. This ability to measure the composition of treatment targets in vivo could enable assessment of tumour hypoxia across several particle therapy fractions, for example, or tracking of calcifications in brain metastases.
A research team headed up by Joao Seco at the German Cancer Research Center (DKFZ) has now demonstrated a technique that uses PGS to determine the elemental composition of irradiated tissues. The approach – called proton and ion beam spectroscopy (PIBS) – uses a new generation of CeBr3 scintillation detectors that measure the entire spectrum of the ion-beam-induced prompt gamma rays.
“The CeBr3 scintillation detectors have similar performance as the LaBr3 counterparts used in the prototype PGS system at Massachusetts General Hospital. Both perform very well in terms of energy and time resolution, which is key for PGS,” explains first author Paulo Martins. “LaBr3 detectors have a slightly better energy resolution. However, they are intrinsically radioactive. Therefore, for prompt gammas below 3 MeV, CeBr3 scintillators may outperform LaBr3 scintillators by about a factor 50 in noise reduction.”
Oxygen levels
Martins and collaborators, also from the Heidelberg Ion-Beam Therapy Center (HIT) and the Max Planck Institute for Nuclear Physics, used beams of protons, helium ions and carbon ions at HIT to perform PIBS. They first investigated sugar-in-water solutions with different oxygen concentrations, irradiating the samples with 90.7 MeV protons.
In the low-energy region, they observed prompt gamma emission at 0.718 MeV that increased with decreasing oxygen concentration or increasing carbon concentration. This energy line results from the excitation of carbon nuclei followed by prompt gamma emission. The high energy region showed peaks at 5.2 and 6.1 MeV, which increased with increasing oxygen concentration, due to the excitation of oxygen nuclei. Irradiating the samples with 92 MeV/u helium ion beams produced similar behaviour.
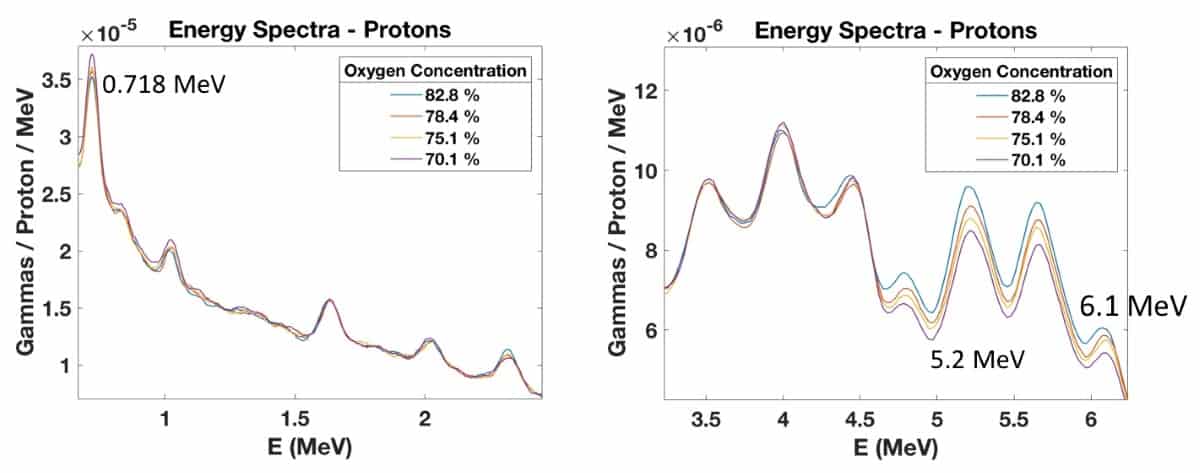
To determine the mass of oxygen irradiated, the researchers placed EBT dosimetry films within the targets along the beam direction. They observed – as seen in previous studies – that the total prompt gammas detected within the 5.2 MeV energy peak increased linearly with the mass of oxygen irradiated.
To confirm the ability of PGS to measure oxygen concentrations deeper in water phantoms, they also irradiated five water/sugar samples with 113.6 MeV protons through 7 cm of water (two water flasks). They observed a clear relationship between increased oxygen concentration and prompt gamma production at 5.2 and 6.1 MeV. The PIBS setup could detect 3% changes in oxygen concentration in the samples.
Tissue surrogates
Next, the team irradiated six water/sugar samples and 12 tissue surrogate inserts with 88.1 MeV/u helium ion and 161.5 MeV/u carbon ion beams. The tissue surrogates contained varying concentrations of oxygen, carbon and calcium, and included five types of bone surrogate with increased calcium concentration, as well as breast, solid-water, muscle and liver surrogates with very low calcium concentrations.
The data from water/sugar samples with higher oxygen concentrations (67.1–88.9%) and tissue surrogate inserts with lower oxygen concentrations (14.9–36.5%) exhibited a logarithmic trend between prompt gamma production at 5.2 MeV and oxygen concentration.

gamma energy spectra resulting from the irradiation of six water solutions and 11 tissue surrogate inserts by helium and carbon beams. (Courtesy: CC BY 4.0/Sci. Rep. 10.1038/s41598-020-63215-0)
The researchers also evaluated whether this relationship held true for other elements, such as calcium. In the low-energy window, irradiating the tissue surrogates with 88.1 MeV/u helium ion beams generated prompt gamma peaks at 1.66 MeV, resulting from calcium reactions. Again, they observed a clear relationship between calcium concentration and prompt gamma production.
The team reported that PIBS could clearly identify calcium concentration changes of 1% between adipose and breast surrogates, and 2% oxygen variations between the various tissue surrogates.

Gamma spectroscopy cuts beam-range uncertainty
“Conversely to PGS, the aim of PIBS is not range verification, but rather determination of the physical and chemical properties of the irradiated targets,” Martins tells Physics World. “This feature is, however, linked to the range in the patient as the body composition will ultimately affect the location where the particles will stop, thus influencing the spatial resolution. By combining PGS with PIBS, other modalities such as CT or PET may be suppressed in future treatment planning, with strong impact on treatment workflows.”
Martins says that future studies will involve in vivo measurements in mice and benchmarking against other state-of-the-art techniques, such as dual-energy CT, PET, nuclear magnetic resonance spectroscopy and dual-energy X-ray absorptiometry. “Such outcomes may be the key to monitor tumour hypoxia in the course of therapy and detect calcifications,” he adds.
The research is published in Scientific Reports.



